Investigation of Histamine Removal by Electrodialysis from the Fermented Fish Sauce and Its Effects on the Flavor
Abstract
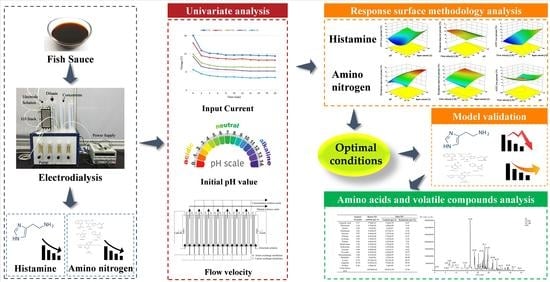
:1. Introduction
2. Materials and Methods
2.1. Fish Sauce Properties
2.2. Electrodialysis Equipment and Procedures
2.3. Univariate Analysis
2.4. Response Surface Methodology (RSM) Analysis
2.5. Quantification of Histamine
2.6. Quantifications of NaCl and ANN
2.7. Analysis of Amino Acid Composition
2.8. Analysis of Volatile Compounds
2.9. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Input Current on Histamine Removal from Fish Sauce
3.2. The Effect of Initial pH Value on Histamine Removal from Fish Sauce
3.3. The Effect of Flow Velocity on Histamine Removal from Fish Sauce
3.4. Optimization of ED Operation Conditions by RSM and Model Validation
3.5. ANN Loss Rate
3.6. Variations in Volatile Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaman, M.Z.; Abu Bakar, F.; Jinap, S.; Bakar, J. Novel Starter Cultures to Inhibit Biogenic Amines Accumulation during Fish Sauce Fermentation. Int. J. Food Microbiol. 2011, 145, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Luan, H.; Bu, Y.; Li, J.; Li, X.; Zhang, Y. Changes in Taste Substances during Fermentation of Fish Sauce and the Correlation with Protease Activity. Food Res. Int. 2021, 144, 110349. [Google Scholar] [CrossRef]
- Ghirri, A.; Bignetti, E. Occurrence and Role of Umami Molecules in Foods. Int. J. Food Sci. Nutr. 2012, 63, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sang, X.; Yan, C.; Zhang, Y.; Bi, J.; Zhang, G.; Hao, H.; Hou, H. Dynamics of Bacterial Composition and Association with Quality Formation and Biogenic Amines Accumulation during Fish Sauce Spontaneous Fermentation. Appl. Environ. Microbiol. 2022, 88, e00690-22. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.Z.; Abdulamir, A.S.; Bakar, F.A. Microbiological, Physicochemical and Health Impact of High Level of Biogenic Amines in Fish Sauce. Am. J. Appl. Sci. 2009, 6, 1199–1211. [Google Scholar]
- Hungerford, J.M. Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Hammes, W.P. Formation of Biogenic Amine in Mayonnaise, Herring and Tuna Fish Salad by Lactobacilli. Int. J. Food Sci. Nutr. 1999, 50, 159–164. [Google Scholar] [CrossRef]
- Evangelista, W.P.; Silva, T.M.; Guidi, L.R.; Tette, P.A.S.; Byrro, R.M.D.; Santiago-Silva, P.; Fernandes, C.; Gloria, M.B.A. Quality Assurance of Histamine Analysis in Fresh and Canned Fish. Food Chem. 2016, 211, 100–106. [Google Scholar] [CrossRef]
- Jiang, W.; He, X.; Yang, H.; Xiang, X.; Hu, S.; Li, S.; Liu, Y. Histamine Reduction by Maillard Reaction with Glucose. Food Control 2017, 82, 136–144. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine Food Poisoning. Histamine Histamine Recept. Health Dis. 2016, 241, 217–235. [Google Scholar] [CrossRef]
- Nazari, M.; Mooraki, N.; Sedaghati, M. Chemical and Microbial Properties of a Fermented Fish Sauce in the Presence of Lactobacillus Plantarum and Paenibacillus Polymyxa. Iran J. Fish Sci. 2021, 20, 663–677. [Google Scholar]
- Alvarez, M.A.; Moreno-Arribas, M.V. The Problem of Biogenic Amines in Fermented Foods and the Use of Potential Biogenic Amine-Degrading Microorganisms as a Solution. Trends. Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Turki, T.; Hamdouni, A. Study of Nitrate Removal from Aqueous Solution by Electrodialysis. Arab. J. Geosci. 2022, 15, 780. [Google Scholar] [CrossRef]
- Lemaire, J.; Blanc, C.-L.; Duval, F.; Théoleyre, M.-A.; Pareau, D. Purification of Pentoses from Hemicellulosic Hydrolysates with Sulfuric Acid Recovery by Using Electrodialysis. Sep. Purif. Technol. 2016, 166, 181–186. [Google Scholar] [CrossRef]
- Luiz, A.; McClure, D.D.; Lim, K.; Coster, H.G.L.; Barton, G.W.; Kavanagh, J.M. Towards a Model for the Electrodialysis of Bio-Refinery Streams. J. Memb Sci. 2019, 573, 320–332. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Q.; Yang, P.; Cong, W. Transport Properties of Amino Acid Ions at Isoelectric Point in Electrodialysis. Sep. Purif. Technol. 2016, 168, 257–264. [Google Scholar] [CrossRef]
- Ma, H.; Yue, S.; Li, H.; Wang, Q.; Tu, M. Recovery of Lactic Acid and Other Organic Acids from Food Waste Ethanol Fermentation Stillage: Feasibility and Effects of Substrates. Sep. Purif. Technol. 2019, 209, 223–228. [Google Scholar] [CrossRef]
- Bak, C.; Yun, Y.-M.; Kim, J.-H.; Kang, S. Electrodialytic Separation of Volatile Fatty Acids from Hydrogen Fermented Food Wastes. Int. J. Hydrogen Energy 2019, 44, 3356–3362. [Google Scholar] [CrossRef]
- Okubo, A.; Noma, S.; Demura, M.; Hayashi, N. Accelerated Production of Reduced-Salt Sardine Fish Sauce under Pressurized Carbon Dioxide, Combining Mild Heating and Proteolysis. Food Sci. Technol. Res. 2022, 28, FSTR. [Google Scholar] [CrossRef]
- Chindapan, N.; Devahastin, S.; Chiewchan, N. Electrodialysis Desalination of Fish Sauce: Electrodialysis Performance and Product Quality. J. Food Sci. 2009, 74, E363–E371. [Google Scholar] [CrossRef]
- Chindapan, N.; Devahastin, S.; Chiewchan, N. Effect of Electrodialysis Pretreatment on Physicochemical Properties and Morphology of Spray-Dried-Fish Sauce Powder. J. Food Eng. 2010, 99, 31–39. [Google Scholar] [CrossRef]
- Chindapan, N.; Devahastin, S.; Chiewchan, N.; Sablani, S.S. Desalination of Fish Sauce by Electrodialysis: Effect on Selected Aroma Compounds and Amino Acid Compositions. J. Food Sci. 2011, 76, S451–S457. [Google Scholar] [CrossRef]
- Jundee, J.; Devahastin, S.; Chiewchan, N. Development and Testing of a Pilot-Scale Electrodialyser for Desalination of Fish Sauce. Procedia Eng. 2012, 32, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Maśliński, C. Histamine and Its Metabolism in Mammals Part I: Chemistry and Formation of Histamine. Agents Actions 1975, 5, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Bongaers, E.; Alenus, J.; Horemans, F.; Weustenraed, A.; Lutsen, L.; Vanderzande, D.; Cleij, T.J.; Troost, F.J.; Brummer, R.-J.; Wagner, P. A MIP-Based Biomimetic Sensor for the Impedimetric Detection of Histamine in Different PH Environments. Phys. Status Solidi 2010, 207, 837–843. [Google Scholar] [CrossRef]
- Taylor, S.L.; Eitenmiller, R.R. Histamine Food Poisoning: Toxicology and Clinical Aspects. CRC Crit. Rev. Toxicol. 1986, 17, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Ackarabanpojoue, Y.; Chindapan, N.; Yoovidhya, T.; Devahastin, S. Electrodialytic Removal of Nitrate from Pineapple Juice: Effect on Selected Physicochemical Properties, Amino Acids, and Aroma Components of the Juice. J. Food Sci. 2015, 80, E998–E1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, M.; Wang, M.; Zhou, G.; Liu, F.; Zhang, Y. Porphyrin Thin-Film Composite Cation Exchange Membranes Enable High Retention of Amino Acids in Electrodialysis. Sep. Purif. Technol. 2021, 279, 119729. [Google Scholar] [CrossRef]
- Box, G.E.; Behnken, D.W. Some New Three Level Designs for the Study of Quantitative Variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Zhao, D.-D.; Liu, J.-H.; Lu, F.; Ding, Y.-T. Physical, Chemical and Microbiological Characteristics of Fermented Surimi with Actinomucor Elegans. LWT—Food Sci. Technol. 2014, 59, 335–341. [Google Scholar] [CrossRef]
- Lu, M.; An, H.; Wang, D. Characterization of Amino Acid Composition in Fruits of Three Rosa Roxburghii Genotypes. Hortic. Plant J. 2017, 3, 232–236. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X. Analysis of Volatile Flavor of Fish Sauce by HS-SPME with GC-MS. J. Anhui Agric. Sci. 2008, 36, 9838–9841. [Google Scholar]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of Polyethyleneimine Coating on Membrane Permselectivity and Desalination Performance during Pilot-Scale Electrodialysis of Reverse Osmosis Concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar] [CrossRef]
- Marder, L.; Bittencourt, S.D.; Zoppas Ferreira, J.; Bernardes, A.M. Treatment of Molybdate Solutions by Electrodialysis: The Effect of PH and Current Density on Ions Transport Behavior. Sep. Purif. Technol. 2016, 167, 32–36. [Google Scholar] [CrossRef]
- Pérez, A.; Andrés, L.J.; Álvarez, R.; Coca, J.; Hill, C.G. Electrodialysis of Whey Permeates and Retentates Obtained by Ultrafiltration. J. Food Process Eng. 1994, 17, 177–190. [Google Scholar] [CrossRef]
- Fidaleo, M.; Moresi, M.; Cammaroto, A.; Ladrange, N.; Nardi, R. Soy Sauce Desalting by Electrodialysis. J. Food Eng. 2012, 110, 175–181. [Google Scholar] [CrossRef]
- Ge, S.; Li, W.; Zhang, Z.; Li, C.; Wang, Y. Desalting of Tobacco Extract Using Electrodialysis. Membr. Water Treat. 2016, 7, 341–353. [Google Scholar] [CrossRef]
- Merkel, A.; Ashrafi, A.M.; Ečer, J. Bipolar Membrane Electrodialysis Assisted PH Correction of Milk Whey. J. Memb. Sci. 2018, 555, 185–196. [Google Scholar] [CrossRef]
- Banasiak, L.J.; Kruttschnitt, T.W.; Schäfer, A.I. Desalination Using Electrodialysis as a Function of Voltage and Salt Concentration. Desalination 2007, 205, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Shaposhnik, V.A.; Eliseeva, T.V. Barrier Effect during the Electrodialysis of Ampholytes. J. Memb. Sci. 1999, 161, 223–228. [Google Scholar] [CrossRef]
- Kattan Readi, O.M.; Kuenen, H.J.; Zwijnenberg, H.J.; Nijmeijer, K. Novel Membrane Concept for Internal PH Control in Electrodialysis of Amino Acids Using a Segmented Bipolar Membrane (SBPM). J. Memb. Sci. 2013, 443, 219–226. [Google Scholar] [CrossRef]
- Wang, M.; Kuang, S.; Wang, X.; Kang, D.; Mao, D.; Qian, G.; Cai, X.; Tan, M.; Liu, F.; Zhang, Y. Transport of Amino Acids in Soy Sauce Desalination Process by Electrodialysis. Membranes 2021, 11, 408. [Google Scholar] [CrossRef]
- Readi, O.M.K.; Mengers, H.J.; Wiratha, W.; Wessling, M.; Nijmeijer, K. On the Isolation of Single Acidic Amino Acids for Biorefinery Applications Using Electrodialysis. J. Memb. Sci. 2011, 384, 166–175. [Google Scholar] [CrossRef]
- Eliseeva, T.; Kharina, A. Desalination of Neutral Amino Acid Solutions in an Electromembrane System. Membranes 2022, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-Y.; Duan, J.-R.; Yu, L.-X.; Xing, X.-H.; Xu, P. Desalination of Glutamine Fermentation Broth by Electrodialysis. Process Biochem. 2006, 41, 716–720. [Google Scholar] [CrossRef]
- Johnson, K.T.; Hill, C.G. Electrodialysis of Raw Whey and Whey Fractionated by Reverse Osmosis and Ultrafiltration. J. Food Sci. 1976, 41, 770–777. [Google Scholar] [CrossRef]
- He, Z.; Gao, X.; Zhang, Y.; Wang, Y.; Wang, J. Revised Spacer Design to Improve Hydrodynamics and Anti-Fouling Behavior in Reverse Electrodialysis Processes. Desalination Water Treat. 2016, 57, 28176–28186. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Sea Water Desalination Using Electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- Poulin, J.-F.; Amiot, J.; Bazinet, L. Impact of Feed Solution Flow Rate on Peptide Fractionation by Electrodialysis with Ultrafiltration Membrane. J. Agric. Food Chem. 2008, 56, 2007–2011. [Google Scholar] [CrossRef]
- Tan, W.; Hao, R.; Liu, Y.; Zhu, G.; Liu, L. Desalination of Graphite Oxide by Electrodialysis. Desalination 2016, 385, 53–57. [Google Scholar] [CrossRef]
- Mourabet, M.; el Rhilassi, A.; el Boujaady, H.; Bennani-Ziatni, M.; Taitai, A. Use of Response Surface Methodology for Optimization of Fluoride Adsorption in an Aqueous Solution by Brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.B.S.; Hamrouni, B. Development of a Predictive Model of the Limiting Current Density of an Electrodialysis Process Using Response Surface Methodology. Membr. Water Treat. 2016, 7, 127–141. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Tian, Z.; Zhao, J.; Wang, J.; Li, P.-F.; Xu, Y. Near-Zero Liquid Discharge and Reclamation Process Based on Electrodialysis Metathesis for High-Salinity Wastewater with High Scaling Potential. Desalination 2022, 525, 115390. [Google Scholar] [CrossRef]
- Jwa, E.; Jeong, N.; Nam, J.-Y.; Han, J.-I. Sustainable Energy Harvesting and On-Site Disinfection of Natural Seawater Using Reverse Electrodialysis. Water Res. 2022, 220, 118681. [Google Scholar] [CrossRef]
- Mohamad Said, K.A.; Mohamed Amin, M.A. Overview on the Response Surface Methodology (RSM) in Extraction Processes. J. Appl. Sci. Process Eng. 2015, 2, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Durant, G.J. Guanidine Derivatives Acting at Histaminergic Receptors. Chem. Soc. Rev. 1985, 14, 375. [Google Scholar] [CrossRef]
- Fitch, C.A.; Platzer, G.; Okon, M.; Garcia-Moreno, E.B.; McIntosh, L.P. Arginine: Its PKa Value Revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.-R.; Yu, S.-H.; Chao, A.-C.; Wu, J.-Y.; Lin, Y.-F.; Lu, K.-Y.; Mi, F.-L. Preparation and Properties of PH-Responsive, Self-Assembled Colloidal Nanoparticles from Guanidine-Containing Polypeptide and Chitosan for Antibiotic Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 9–20. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Wang, Z.; Cai, S.; Zhu, B.; Dong, X. Recent Advances in Fishy Odour in Aquatic Fish Products, from Formation to Control. Int. J. Food Sci. Technol. 2021, 56, 4959–4969. [Google Scholar] [CrossRef]
- Pham, A.J.; Schilling, M.W.; Yoon, Y.; Kamadia, V.V.; Marshall, D.L. Characterization of Fish Sauce Aroma-Impact Compounds Using GC-MS, SPME-Osme-GCO, and Stevens’ Power Law Exponents. J. Food Sci. 2008, 73, C268–C274. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.N.; Man, Y.C.; Mustafa, S.; Manap, Y.A. Tentative Identification of Volatile Flavor Compounds in Commercial Budu, a Malaysian Fish Sauce, Using GC-MS. Molecules 2012, 17, 5062–5080. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The Role of Bacterial Fermentation in Lipolysis and Lipid Oxidation in Harbin Dry Sausages and Its Flavour Development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Sakpetch, P.; Benchama, O.; Masniyom, P.; Salaipeth, L.; Kanjan, P. Physicochemical Characteristics and Flavor Profiles of Fermented Fish Sauce (Budu) during Fermentation in Commercial Manufacturing Plant. J. Food Sci. Technol. 2022, 59, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y.; Endo, K.; Shimoda, M. Identification of Distinctive Volatile Compounds in Fish Sauce. J. Agric. Food Chem. 2002, 50, 5412–5416. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, J.-Y.; Liu, S.-L.; Wang, Y.; Ding, Y.-T. Chemical, Microbiological and Sensory Changes of Dried Acetes Chinensis during Accelerated Storage. Food Chem. 2011, 127, 159–168. [Google Scholar] [CrossRef]
- Udomsil, N.; Chen, S.; Rodtong, S.; Yongsawatdigul, J. Improvement of Fish Sauce Quality by Combined Inoculation of Tetragenococcus Halophilus MS33 and Virgibacillus Sp. SK37. Food Control 2017, 73, 930–938. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Duan, T.; Jiang, J.; Yang, X.; Rong, M. Research Progress of Selective Oxidation of Alcohols to Aldehydes and Ketones by Electrocatalysis. J. Mol. Catal. 2021, 35, 583–594. [Google Scholar]






| Variables | Symbols | Levels | |||
|---|---|---|---|---|---|
| Coded | Uncoded | −1 | 0 | 1 | |
| Input current (A) | X1 | x1 | 4 | 5 | 6 |
| pH | X2 | x2 | 3.0 | 4.0 | 5.0 |
| Flow velocity (L∙h−1) | X3 | x3 | 20 | 30 | 40 |
| Experiment No. | Input Current (A) (X1) | pH (X2) | Flow Velocity (L∙h−1) (X3) | Histamine Removal (%) (Y1) | ANN Loss (%) (Y2) |
|---|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 53.67 | 18.27 |
| 2 | 1 | 1 | 0 | 34.63 | 20.27 |
| 3 | 0 | 0 | 0 | 48.57 | 16.60 |
| 4 | 0 | 1 | −1 | 36.25 | 20.09 |
| 5 | 0 | 0 | 0 | 46.56 | 15.31 |
| 6 | −1 | −1 | 0 | 34.01 | 16.05 |
| 7 | −1 | 0 | 1 | 35.42 | 12.78 |
| 8 | 0 | 0 | 0 | 47.32 | 16.19 |
| 9 | 1 | −1 | 0 | 55.16 | 22.38 |
| 10 | −1 | 0 | −1 | 31.45 | 15.14 |
| 11 | −1 | 1 | 0 | 28.41 | 14.33 |
| 12 | 1 | 0 | 1 | 55.34 | 17.00 |
| 13 | 1 | 0 | −1 | 48.65 | 20.87 |
| 14 | 0 | −1 | −1 | 45.74 | 21.65 |
| 15 | 0 | 1 | 1 | 36.59 | 17.07 |
| Values | Histamine Reductio Rate | ANN Reduction Rate |
|---|---|---|
| R2 | 0.9872 | 0.9853 |
| Adj R2 | 0.9643 | 0.9588 |
| Adeq precision | 20.918 | 19.918 |
| Model F-value | 43.01 a | 37.22 a |
| Lack of fit F-value | 4.08 b | 0.64 b |
| Pure error | 1.03 | 0.43 |
| Optimum Condition | Coded Levels | Actual Levels |
|---|---|---|
| Input current | 0.1 | 5.1 A |
| pH | −0.2 | 3.7 |
| Flow velocity | 1 | 40 L∙h−1 |
| Responses | Predicted values | Experiment values |
| Histamine reduction rate | 52.62% | 53.41% ± 0.41% |
| Histamine content after ED (mg/100 mL) | 38.74 | 38.00 ± 0.33 |
| ANN reduction rate | 15.72% | 15.46% ± 0.28% |
| ANN content after ED (g/100 mL) | 1.035 | 1.06 ± 0.00 |
| Salt reduction rate after ED | - | 56.23 ± 0.1% |
| Isoelectric Point | Before ED Content (g∙L−1) | After ED | Reduction Rate (%) | |
|---|---|---|---|---|
| Content (g∙L−1) | ||||
| Aspartic acid | 2.77 | 7.96 ± 0.11 d | 7.62 ± 0.19 d | 4.27 |
| Threonine | 6.16 | 3.79 ± 0.03 h | 3.48 ± 0.09 g | 8.18 |
| Serine | 5.68 | 3.9 ± 0.05 h | 3.6 ± 0.07 g | 7.69 |
| Glutamic acid | 3.22 | 9.01 ± 0.07 c | 8.17 ± 0.11 c | 9.32 |
| Glycine | 5.97 | 2.64 ± 0.02 k | 2.37 ± 0.04 i | 10.23 |
| Alanine | 6.00 | 6.01 ± 0.04 e | 5.52 ± 0.07 e | 8.15 |
| Cysteine | 5.05 | 1.78 ± 0.01 m | 1.67 ± 0.03 j | 6.18 |
| Proline | 5.96 | 4.82 ± 0.03 c | 4.47 ± 0.06 b | 7.26 |
| Methionine | 5.74 | 2.59 ± 0.02 k | 2.39 ± 0.03 i | 7.72 |
| Isoleucine | 6.02 | 3.17 ± 0.04 j | 2.87 ± 0.04 h | 9.46 |
| Leucine | 5.98 | 4.99 ± 0.05 f | 4.51 ± 0.11 f | 9.62 |
| Tyrosine | 5.68 | 0.32 ± 0.01 o | 0.26 ± 0.05 k | 18.75 |
| Phenylalanine | 5.48 | 2.27 ± 0.03 l | 2.04 ± 0.07 i | 10.13 |
| Histidine | 7.59 | 3.4 ± 0.06 i | 2.35 ± 0.16 i | 30.88 |
| Lysine | 9.74 | 31.02 ± 0.21 a | 24.7 ± 0.49 a | 20.37 |
| Arginine | 10.76 | 13.63 ± 0.16 b | 9.55 ± 0.34 bc | 29.93 |
| Proline | 6.30 | 1.58 ± 0.01 g | 1.4 ± 0.09 j | 11.39 |
| Total amino acids | 102.88 ± 0.41 | 86.97 ± 1.07 | 15.46 |
| Compound Name | Relative Content (%) | Significant Differences before and after ED | Relative Change | Odor Description | |
|---|---|---|---|---|---|
| Before ED | After ED | ||||
| Alcohol | 5.54 | 2.75 | |||
| Cyclopentanol | 0.01 ± 0.01 d | 0.01 ± 0.01 f | A, A (p > 0.05) | ↘ | |
| 2-ethylhexanol | 1.96 ± 0.6 d | 1.78 ± 0.16 cdef | A, A (p > 0.05) | ↘ | Floral, perfume |
| phenethyl alcohol | 3.35 ± 0.63 cd | 0.96 ± 0.16 ef | A, B (p < 0.05) | ↘↘ | Floral, perfume |
| 2-furfuryl alcohol | 0.20 ± 0.10 d | 0.00 ± 0.00 f | A, A (p > 0.05) | ↘↘ | Oily, burnt sugar |
| Aldehyde | 68.27 | 59.58 | |||
| octanal | 0.66 ± 0.37 d | 0.66 ± 0.13 ef | A, A (p > 0.05) | - | mint, floral, fruit, resin |
| 3-methyl n-butyl aldehyde | 1.39 ± 0.42 d | 0.69 ± 0.27 ef | A, A (p > 0.05) | ↘↘ | |
| 2-methylpropyl aldehyde | 5.22 ± 1.24 cd | 3.06 ± 0.39 cdef | A, A (p > 0.05) | ↘ | |
| 3-methyl n-butyl aldehyde | 21.37 ± 2.27 a | 14.03 ± 3.82 a | A, A (p > 0.05) | ↘ | Almond, nutty, buttery |
| 2-methyl n-butyl aldehyde | 10.71 ± 5.81 b | 9.70 ± 2.02 b | A, A (p > 0.05) | ↘ | Nutty, buttery, oily |
| 2, 3-dimethylglutaraldehyde | 0.24 ± 0.10 d | 1.48 ± 0.12 def | A, A (p > 0.05) | ↗↗ | |
| 2-ethyl-4-glutenal | 0.00 ± 0.00 d | 9.44 ± 1.32 b | A, B (p < 0.05) | ↗↗ | |
| methylthiopropanal | 18.21 ± 5.41 a | 13.91 ± 1.93 a | A, A (p > 0.05) | ↘ | Boiled potato |
| benzaldehyde | 2.82 ± 0.08 cd | 1.75 ± 0.74 cdef | A, B (p < 0.05) | ↘ | Almond, burnt sugar, sweet |
| phenylacetaldehyde | 4.00 ± 1.19 cd | 2.37 ± 0.46 cdef | A, A (p > 0.05) | ↘ | Beer not fresh |
| phenylglyoxal | 1.33 ± 0.19 d | 1.20 ± 0.07 ef | A, A (p > 0.05) | ↘ | |
| pelargonic aldehyde | 2.31 ± 0.22 cd | 1.29 ± 0.42 ef | A, A (p > 0.05) | ↘ | Green, grassy, moss |
| Ketone | 2.41 | 6.19 | |||
| butanone | 2.17 ± 1.02 cd | 5.38 ± 1.40 c | A, A (p > 0.05) | ↗↗ | cheesy |
| 5-ethyl-2 (5H) -furanone | 0.24 ± 0.02 d | 0.81 ± 0.25 ef | A, B (p < 0.05) | ↗↗ | cheesy |
| Carboxylic acid | 9.90 | 19.32 | |||
| N-methyl taurine | 0.08 ± 0.03 d | 0.57 ± 0.36 ef | A, A (p > 0.05) | ↗↗ | |
| 4-methylvaleric acid | 0.47 ± 0.22 d | 0.20 ± 0.11 f | A, A (p > 0.05) | ↘ | Pungent sour |
| hexanoic acid | 1.65 ± 0.28 d | 9.44 ± 3.55 b | A, A (p > 0.05) | ↗↗ | |
| 3-methylbutyric acid | 4.15 ± 0.47 cd | 5.35 ± 0.32 cd | A, A (p > 0.05) | ↗ | Dirty socks, sweaty, cheesy |
| 2-methylbutyric acid | 1.71 ± 0.15 d | 2.20 ± 0.06 cdef | A, A (p > 0.05) | ↗ | Cheesy |
| 2-methylhexanoic acid | 1.79 ± 0.79 d | 1.47 ± 0.09 def | A, B (p < 0.05) | ↘ | |
| n-nonanoic acid | 0.05 ± 0.03 d | 0.08 ± 0.01 f | A, A (p > 0.05) | ↗ | |
| Pyrazine | 2.28 | 5.79 | |||
| methylpyrazine | 0.45 ± 0.09 d | 0.11 ± 0.04 f | A, A (p > 0.05) | ↘↘ | |
| 2, 6-dimethylpyrazine | 1.33 ± 0.57 d | 4.43 ± 1.87 cde | A, A (p > 0.05) | ↗↗ | Cooked rice, sweet |
| 2-ethyl-6-methylpyrazine | 0.50 ± 0.30 d | 1.26 ± 0.40 ef | A, A (p > 0.05) | ↗↗ | |
| Others | 11.60 | 6.37 | |||
| Dimethyl disulfide | 3.13 ± 0.79 cd | 2.25 ± 0.26 cdef | A, A (p > 0.05) | ↘ | |
| 4-methyl-pyrimidine | 0.37 ± 0.05 d | 0.95 ± 0.29 ef | A, A (p > 0.05) | ↗↗ | |
| 2, 4-di-tert-butylphenol | 0.78 ± 0.14 d | 0.23 ± 0.15 f | A, A (p > 0.05) | ↘↘ | |
| 2-acetylpyrrole | 7.19 ± 2.08 bc | 2.73 ± 0.95 cdef | A, A (p > 0.05) | ↘↘ | |
| acetophenone | 0.06 ± 0.04 d | 0.10 ± 0.00 f | A, B (p < 0.05) | ↗ | |
| 2-methyl-naphthalene | 0.09 ± 0.04 d | 0.10 ± 0.00 f | A, B (p < 0.05) | ↗ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Qiu, M.; Tang, X.; Zheng, R.; Zhou, X. Investigation of Histamine Removal by Electrodialysis from the Fermented Fish Sauce and Its Effects on the Flavor. Foods 2023, 12, 1325. https://doi.org/10.3390/foods12061325
Ding Y, Qiu M, Tang X, Zheng R, Zhou X. Investigation of Histamine Removal by Electrodialysis from the Fermented Fish Sauce and Its Effects on the Flavor. Foods. 2023; 12(6):1325. https://doi.org/10.3390/foods12061325
Chicago/Turabian StyleDing, Yicheng, Mengting Qiu, Xiaoling Tang, Renchao Zheng, and Xuxia Zhou. 2023. "Investigation of Histamine Removal by Electrodialysis from the Fermented Fish Sauce and Its Effects on the Flavor" Foods 12, no. 6: 1325. https://doi.org/10.3390/foods12061325