The Introduction of Allochthonous Olive Variety and Super High-Density System in the Abruzzo Region: A Study on Olive Oil Quality
Abstract
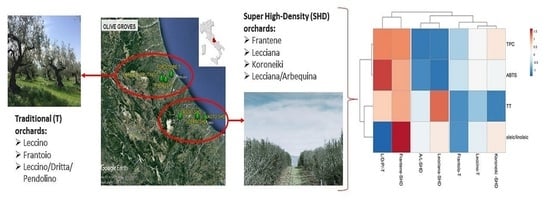
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Oils
2.2. Olive Oils Chemical Characterization
2.3. Phenol Extraction
2.4. Total Phenolic Compounds
2.5. Antioxidant Activity
2.6. Total Tocopherols
2.7. Phenolic Profile
2.8. Statistical Analysis
3. Results and Discussion
3.1. Free Acidity, Peroxide Value, K232, K270
3.2. Quality and Functional Parameters: Total Phenolic Content, Antioxidant Activity and Total Tocopherol
3.3. Phenolic Profile
3.4. Fatty acid Profile
3.5. Cluster Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lombardo, L.; Farolfi, C.; Tombesi, S.; Novelli, E.; Capri, E. Development of a sustainability technical guide for the Italian olive oil supply chain. Sci. Total Environ. 2022, 820, 153332. [Google Scholar] [CrossRef]
- Flamminii, F.; Marone, E.; Neri, L.; Pollastri, L.; Cichelli, A.; Di Mattia, C.D. The Effect of Harvesting Time on Olive Fruits and Oils Quality Parameters of Tortiglione and Dritta Olive Cultivars. Eur. J. Lipid Sci. Technol. 2021, 123, 2000382. [Google Scholar] [CrossRef]
- Di Serio, M.G.; Giansante, L.; Del Re, P.; Pollastri, L.; Panni, F.; Valli, E.; Di Giacinto, L. Characterization of ‘Olivastro di Bucchianico cv’ extra virgin olive oils and its recognition by HS-GC-IMS. J. Sci. Food Agric. 2021, 101, 6074–6082. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, W.; Mascio, I.; Squeo, G.; Gadaleta, S.; Flamminii, F.; Conte, P.; Di Mattia, C.D.; Piga, A.; Caponio, F.; Montemurro, C. Bioactive potential of minor italian olive genotypes from Apulia, Sardinia and Abruzzo. Foods 2021, 10, 1371. [Google Scholar] [CrossRef]
- Polari, J.J.; Mori, M.; Wang, S.C. Virgin Olive Oils from Super-High-Density Orchards in California: Impact of Cultivar, Harvest Time, and Crop Season on Quality and Chemical Composition. Eur. J. Lipid Sci. Technol. 2021, 123, 2000180. [Google Scholar] [CrossRef]
- Farinelli, D.; Tombesi, S. Performance and oil quality of ‘Arbequina’ and four Italian olive cultivars under super high density hedgerow planting system cultivated in central Italy. Sci. Hortic. 2015, 192, 97–107. [Google Scholar] [CrossRef]
- Tombesi, A.; Proietti, P.; Iacovelli, G.; Tombesi, S.; Farinelli, D. Vegetative and productive behaviour of four olive Italian cultivars and “Arbequina” according to super intensive olive training system in central Italy. Acta Hortic. 2011, 924, 211–218. [Google Scholar] [CrossRef]
- Tombesi, S.; Tombesi, A.; Molfese, M.; Cipolletti, M.; Visco, T. Evaluation of four cultivars regarding their suitability in high-intensity olive orchards. Acta Hortic. 2011, 924, 321–326. [Google Scholar] [CrossRef]
- Alfei, B.; Paoletti, A.; Pannelli, G.; Santinelli, A.; Rosati, A. Agronomic and Qualitative Evaluation of New Olive Genotypes Selected in Central Italy. Adv. Hortic. Sci. 2008, 22, 1000–1006. [Google Scholar] [CrossRef]
- Camposeo, S.; Vivaldi, G.A.; Montemurro, C.; Fanelli, V.; Canal, M.C. Lecciana, a New Low-Vigour Olive Cultivar Suitable for Super High Density Orchards and for Nutraceutical EVOO Production. Agronomy 2021, 11, 2154. [Google Scholar] [CrossRef]
- Pattara, C.; Russo, C.; Antrodicchia, V.; Cichelli, A. Carbon footprint as an instrument for enhancing food quality: Overview of the wine, olive oil and cereals sectors. J. Sci. Food Agric. 2017, 97, 396–410. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, S.; Vivaldi, G.A.; Russo, G.; Melucci, F.M. Intensification in Olive Growing Reduces Global Warming Potential under Both Integrated and Organic Farming. Sustainability 2022, 14, 6389. [Google Scholar] [CrossRef]
- European Commission. Commission Delegate Regulation (EU) 2022/2104. Off. J. Eur. Union 2022, 65, 1. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2022/2105. Off. J. Eur. Union 2022, 65, 23. [Google Scholar]
- Del Carlo, M.; Sacchetti, G.; Di Mattia, C.; Compagnone, D.; Mastrocola, D.; Liberatore, L.; Cichelli, A. Contribution of the phenolic fraction to the antioxidant activity and oxidative stability of olive oil. J. Agric. Food Chem. 2004, 52, 4072–4079. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/69595.html (accessed on 11 October 2022).
- Oliva, E.; Viteritti, E.; Fanti, F.; Eugelio, F.; Pepe, A.; Palmieri, S.; Sergi, M.; Compagnone, D. Targeted and semi-untargeted determination of phenolic compounds in plant matrices by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1651, 462315. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Benito, M.; Lasa, J.M.; Gracia, P.; Oria, R.; Abenoza, M.; Varona, L.; Sánchez-Gimeno, A.C. Olive oil quality and ripening in super-high-density Arbequina orchard. J. Sci. Food Agric. 2013, 93, 2207–2220. [Google Scholar] [CrossRef] [Green Version]
- Yousfi, K.; Cert, R.M.; García, J.M. Changes in quality and phenolic compounds of virgin olive oils during objectively described fruit maturation. Eur. Food Res. Technol. 2006, 223, 117–124. [Google Scholar] [CrossRef]
- Kiritsakis, A.; Nanos, G.D.; Polymenopulos, Z.; Thomai, T.; Sfakiotakis, E.M. Effect of fruit storage conditions on olive oil quality. J. Am. Oil Chem. Soc. 1998, 75, 721–724. [Google Scholar] [CrossRef]
- Gömez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Del Carlo, M.; Compagnone, D.; Cichelli, A. Effects of fly attack (Bactrocera oleae) on the phenolic profile and selected chemical parameters of olive oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Nenadis, N.; Mastralexi, A.; Tsimidou, M.Z. Physicochemical Characteristics and Antioxidant Potential of the Greek PDO and PGI Virgin Olive Oils (VOOs). Eur. J. Lipid Sci. Technol. 2019, 121, 1800172. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Ouzounidou, G.; Tsantili, E. Fruit Ripening, Antioxidants and Oil Composition in Koroneiki Olives (Olea europea L.) at Different Maturity Indices. Agronomy 2021, 11, 122. [Google Scholar] [CrossRef]
- Grilo, F.; Sedaghat, S.; Di Stefano, V.; Sacchi, R.; Caruso, T.; Lo Bianco, R. Tree Planting Density and Canopy Position Affect ‘Cerasuola’ and ‘Koroneiki’ Olive Oil Quality. Horticulturae 2021, 7, 11. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Connor, D.J.; Gómez-Del-campo, M. Response of Oil Production and Quality to Hedgerow Design in Super-High-Density Olive cv. Arbequina Orchards. Agronomy 2021, 11, 1632. [Google Scholar] [CrossRef]
- Motilva, M.J.; Tovar, M.J.; Romero, M.P.; Alegre, S.; Girona, J. Evolution of oil accumulation and polyphenol content in fruits of olive tree (Olea europaea L.) related to different irrigation strategies. Acta Hortic. 2002, 586, 345–348. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; de Torres, A.; Moya, M.; Espínola, F. Characterization of Olive Oils from Superintensive Crops with Different Ripening Degree, Irrigation Management, and Cultivar: (Arbequina, Koroneiki, and Arbosana). Eur. J. Lipid Sci. Technol. 2019, 121, 1800360. [Google Scholar] [CrossRef]
- Roselli, L.; Clodoveo, M.L.; Corbo, F.; De Gennaro, B. Are health claims a useful tool to segment the category of extra-virgin olive oil? Threats and opportunities for the Italian olive oil supply chain. Trends Food Sci. Technol. 2017, 68, 176–181. [Google Scholar] [CrossRef]
- Rodríguez-Juan, E.; Martínez Román, F.; Sánchez-García, A.; Fernández-Bolaños, J.; García-Borrego, A. From Low-Quality Olive Oils to Valuable Bioactive Compounds: Obtaining Oleacein and Oleocanthal from Olive Oils Intended for Refining. J. Agric. Food Chem. 2022, 70, 333–342. [Google Scholar] [CrossRef]
- Pacetti, D.; Boarelli, M.C.; Giovannetti, R.; Ferraro, S.; Conti, P.; Alfei, B.; Caprioli, G.; Ricciutelli, M.; Sagratini, G.; Fedeli, D.; et al. Chemical and sensory profiling of monovarietal extra virgin olive oils from the italian marche region. Antioxidants 2020, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajoub, A.; Medina-Rodríguez, S.; Olmo-García, L.; Ajal, E.A.; Monasterio, R.P.; Hanine, H.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach. Int. J. Mol. Sci. 2016, 18, 52. [Google Scholar] [CrossRef]
- Sánchez De Medina, V.; Priego-Capote, F.; De Castro, M.D.L. Characterization of monovarietal virgin olive oils by phenols profiling. Talanta 2015, 132, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.; Kiss, A.K.; Naruszewicz, M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Selvaggini, R.; Servili, M.; Urbani, S.; Esposto, S.; Taticchi, A.; Montedoro, G.F. Evaluation of phenolic compounds in virgin olive oil by direct injection in high-performance liquid chromatography with fluorometric detection. J. Agric. Food Chem. 2006, 54, 2832–2838. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.H.C.; De Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Beauchamp, G.; Keast, R.; Morel, D.; Lin, J.; Pika, J. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, V.; Miho, H.; Melliou, E.; Magiatis, P.; Priego-Capote, F.; Luque de Castro, M.D. Quantitative method for determination of oleocanthal and oleacein in virgin olive oils by liquid chromatography–tandem mass spectrometry. Talanta 2017, 162, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Xu, C.; Deng, Y.; Wen, B.; Xie, P.; Huang, L. Effect of geographical location and soil fertility on main phenolic compounds and fatty acids compositions of virgin olive oil from Leccino cultivar in China. Food Res. Int. 2022, 157, 111207. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.M.; Alcalá, S.; Ocaña, M.T.; De Torres, A.; Espínola, F.; Moya, M. Elaboration of extra-virgin olive oils rich in oleocanthal and oleacein: Pilot plant’s proposal. Eur. Food Res. Technol. 2020, 246, 1459–1468. [Google Scholar] [CrossRef]
- Mafrica, R.; Piscopo, A.; De Bruno, A.; Poiana, M. Effects of Climate on Fruit Growth and Development on Olive Oil Quality in Cultivar Carolea. Agriculture 2021, 11, 147. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Contrasting patterns of fatty acid composition and oil accumulation during fruit growth in several olive varieties and locations in a non-Mediterranean region. Eur. J. Agron. 2014, 52, 237–246. [Google Scholar] [CrossRef]
- Hernández, L.M.; Padilla, M.N.; Mancha, M.; Martínez-Rivas, J.M. Expression analysis identifies FAD2-2 as the olive oléate desaturase gene mainly responsible for the linoleic acid content in virgin olive oil. J. Agric. Food Chem. 2009, 57, 6199–6206. [Google Scholar] [CrossRef]
- Di Lecce, G.; Piochi, M.; Pacetti, D.; Frega, N.G.; Bartolucci, E.; Scortichini, S.; Fiorini, D. Eleven monovarietal extra virgin olive oils from olives grown and processed under the same conditions: Effect of the cultivar on the chemical composition and sensory traits. Foods 2020, 9, 904. [Google Scholar] [CrossRef]
- Proietti, P.; Famiani, F.; Tombesi, A. Gas Exchange in Olive Fruit. Photosynthetica 1999, 36, 423–432. [Google Scholar] [CrossRef]
- Gregoriou, C.; Ponitikis, K.; Vemmos, S.N. Effect of shading on yield, mophological characteristics of the fruit, oil content and ratio of fatty acids of oil of olive variety ‘Koroneiki’. In V International Symposium on Olive Growing; Özkaya, M.T., Lavee, S., Ferguson, L., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2008. [Google Scholar]
- Diaz-Espejo, A.; Hafidi, B.; Fernandez, J.E.; Palomo, M.J.; Sinoquet, H. Transpiration and photosynthesis of the olive tree: A model approach. In IV International Symposium on Olive Growing; International Society for Horticultural Science: Leuven, Belgium, 2002; Volume 586, pp. 457–460. [Google Scholar]




| Orchard Type | Variety | Year | Sample Code | Location | Altitude (m) | DfS (km) | Average T (°C) | Rainfall (mm) |
|---|---|---|---|---|---|---|---|---|
| Traditional | Frantoio | 2020 | B2 | Loreto Aprutino | 170 | 18 | 15.8 | 620 |
| 2021 | C6 | 15.8 | 412 | |||||
| L/D/P | 2020 | B14 | Spoltore | 80 | 6 | 16.2 | 440 | |
| 2021 | C1 | 16.4 | 343 | |||||
| Leccino | 2020 | B10 | Pianella | 169 | 15 | 16.6 | 563 | |
| 2021 | C11 | 16.3 | 423 | |||||
| Super High-density | Frantene | 2020 | B3 | Vasto | 105 | 2 | 17.0 | 406 |
| 2021 | C13 | 16.7 | 515 | |||||
| A/L | 2020 | B4 | Casoli | 104 | 20 | 15.7 | 395 | |
| 2021 | C2 | 15.6 | 275 | |||||
| Lecciana | 2020 | B12 | Scerni | 209 | 10 | 17.6 | 499 | |
| 2021 | C5 | 17.4 | 434 | |||||
| Koroneiki | 2020 | B13 | Scerni | 209 | 10 | 17.6 | 499 | |
| 2021 | C4 | 17.4 | 434 |
| Sample | Variety | Year | FA (Oleic Acid %) | PV (mEq O2/kg) | K232 | K270 |
|---|---|---|---|---|---|---|
| B2 | Frantoio | 2020 | 0.17 ± 0.02 c | 4.5 ± 0.54 bc | 1.68 ± 0.17 ab | 0.04 ± 0.01 c |
| C6 | 2021 | 0.34 ± 0.04 a | 4.5 ± 0.54 bc | 1.40 ± 0.14 abc | 0.10 ± 0.01 ab | |
| B14 | L/D/P | 2020 | 0.20 ± 0.02 bc | 8.0 ± 0.96 a | 1.65 ± 0.17 abc | 0.05 ± 0.01 c |
| C1 | 2021 | 0.34 ± 0.04 a | 5.0 ± 0.60 bc | 1.54 ± 0.15 abc | 0.10 ± 0.01 ab | |
| B10 | Leccino | 2020 | 0.34 ± 0.04 a | 4.0 ± 0.50 c | 1.36 ± 0.14 abc | 0.04 ± 0.01 c |
| C11 | 2021 | 0.33 ± 0.04 a | 4.7 ± 0.56 bc | 1.37 ± 0.14 abc | 0.04 ± 0.01 c | |
| B3 | Frantene | 2020 | 0.35 ± 0.04 a | 5.0 ± 0.6 bc | 1.52 ± 0.15 abc | 0.04 ± 0.01 c |
| C13 | 2021 | 0.15 ± 0.02 c | 5.0 ± 0.6 bc | 1.25 ± 0.13 bc | 0.10 ± 0.01 b | |
| B4 | A/L | 2020 | 0.17 ± 0.02 c | 5.0 ± 0.5 bc | 1.54 ± 0.15 abc | 0.04 ± 0.01 c |
| C2 | 2021 | 0.19 ± 0.02 c | 4.2 ± 0.60 c | 1.23 ± 0.12 c | 0.12 ± 0.01 a | |
| B12 | Lecciana | 2020 | 0.35 ± 0.04 a | 4.0 ± 0.48 c | 1.72 ± 0.17 a | 0.04 ± 0.01 c |
| C5 | 2021 | 0.13 ± 0.02 c | 4.0 ± 0.48 c | 1.34 ± 0.13 abc | 0.10 ± 0.01 ab | |
| B13 | Koroneiki | 2020 | 0.28 ± 0.03 ab | 4.5 ± 0.54 bc | 1.52 ± 0.15 abc | 0.05 ± 0.01 c |
| C4 | 2021 | 0.37 ± 0.04 a | 6.0 ± 0.72 b | 1.24 ± 0.12 c | 0.04 ± 0.01 c |
| Sample | Variety | Year | OH-Tyrosol | Tyrosol | Diosmetin | Luteolin | Apigenin | Oleacein | Oleocanthal | TPC | ABTS | TT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 | Frantoio | 2020 | 2.0 ± 0.2 bcd | 6.2 ± 0.7 ab | 0.9 ± 0.1 ef | 3.1 ± 0.4 cdef | 1.1 ± 0.1 c | 80.8 ± 9.7 de | 170.6 ± 20.5 cd | 310 ± 37 bcd | 420 ± 50 abc | 208 ± 25 def |
| C6 | 2021 | 5.9 ± 0.7 a | 6.3 ± 0.8 a | 0.6 ± 0.1 f | 3.3 ± 0.4 bcde | 0.6 ± 0.7 defg | 51.5 ± 6.2 e | 76.8 ± 9.2 fgh | 140 ± 17 f | 220 ± 26 de | 228 ± 27 cdef | |
| B14 | L/D/P | 2020 | 0.7 ± 0.1 ef | 5.1 ± 0.6 abc | 1.4 ± 0.2 de | 1.0 ± 0.1 g | 0.5 ± 0.1 efg | 168.7 ± 20.2 c | 329.6 ± 39.6 a | 320 ± 38 bc | 512 ± 61 ab | 285 ± 34 bcde |
| C1 | 2021 | 1.1 ± 0.1 def | 2.4 ± 0.3 ef | 0.7 ± 0.1 f | 1.9 ± 0.2 fg | 0.3 ± 0.0 g | 101.6 ± 12.2 d | 141.9 ± 17.0 cdef | 400 ± 48 ab | 520 ± 62 ab | 325 ± 39 abc | |
| B10 | Leccino | 2020 | 1.3 ± 0.1 def | 4.6 ± 0.6 bcd | 1.4 ± 0.2 de | 2.7 ± 0.3 def | 1.0 ± 0.1 c | 74.9 ± 9.0 de | 198.4 ± 23.8 bc | 286 ± 34 cde | 400 ± 48 abc | 179 ± 21 f |
| C11 | 2021 | 2.6 ± 0.3 bc | 6.4 ± 0.8 a | 0.9 ± 0.1 ef | 4.1 ± 0.5 bc | 0.6 ± 0.1 defg | 70.2 ± 8.4 de | 103.9 ± 12.5 efg | 270 ± 32 cde | 350 ± 42 cd | 300 ± 36 bcd | |
| B3 | Frantene | 2020 | 0.7 ± 0.1 ef | 5.0 ± 0.6 abc | 2.6 ± 0.3 b | 2.3 ± 0.3 efg | 0.9 ± 0.1 cd | 349.9 ± 42.0 a | 152.7 ± 18.3 cde | 267 ± 32 cde | 390 ± 47 bc | 349 ± 42 ab |
| C13 | 2021 | 2.9 ± 0.4 b | 5.4 ± 0.7 abc | 1.6 ± 0.2 cd | 2.9 ± 0.4 cdef | 0.5 ± 0.1 fg | 63.5 ± 7.6 de | 28.8 ± 3.5 h | 450 ± 54 a | 530 ± 64 a | 285 ± 34 bcde | |
| B4 | A/L | 2020 | 3.1 ± 0.4 b | 4.8 ± 0.8 abcd | 3.5 ± 0.4 a | 4.5 ± 0.5 b | 1.5 ± 0.2 b | 79.9 ± 9.6 de | 256.1 ± 30.7 b | 145 ± 17 f | 250 ± 30 de | 245 ± 29 cdef |
| C2 | 2021 | 1.4 ± 0.2 def | 1.3 ± 0.2 f | 1.2 ± 0.2 def | 4.2 ± 0.5 bc | 0.8 ± 0.1 cde | 34.1 ± 4.1 e | 72.5 ± 8.7 gh | 200 ± 24 ef | 240 ± 29 de | 280 ± 34 bcde | |
| B12 | Lecciana | 2020 | 0.5 ± 0.1 f | 3.2 ± 0.4 de | 1.7 ± 0.2 cd | 3.1 ± 0.4 cdef | 2.0 ± 0.2 a | 33.4 ± 4.0 e | 109.5 ± 13.1 defg | 136 ± 16 f | 230 ± 28 de | 402 ± 48 a |
| C5 | 2021 | 5.2 ± 0.6 a | 4.0 ± 0.5 cde | 2.5 ± 0.3 b | 8.1 ± 0.9 a | 1.1 ± 0.1 c | 102.2 ± 12.3 d | 123.0 ± 14.8 defg | 210 ± 25 def | 230 ± 28 de | 266 ± 32 bcdef | |
| B13 | Koroneiki | 2020 | 1.7 ± 0.2 cde | 4.9 ± 0.6 abc | 2.1 ± 0.3 bc | 2.2 ± 0.3 efg | 0.6 ± 0.1 defg | 228.7 ± 27.4 b | 351.9 ± 42.2 a | 448 ± 54 a | 520 ± 62 ab | 190 ± 23 ef |
| C4 | 2021 | 6.3 ± 0.8 a | 4.3 ± 0.5 cd | 1.6 ± 0.2 cd | 4.0 ± 0.5 bcd | 0.7 ± 0.1 def | 52.2 ± 6.3 e | 61.6 ± 7.4 gh | 154 ± 18 f | 212 ± 25 e | 239 ± 29 cdef |
| B2 | C6 | B14 | C1 | B10 | C11 | B3 | C13 | B4 | C2 | B12 | C5 | B13 | C4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frantoio | L/D/P | Leccino | Frantene | A/L | Lecciana | Koroneiki | ||||||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| Miristic acid (C14:0) | 0.01 b | 0.03 ab | 0.01 b | 0.02 ab | 0.01 b | 0.03 ab | 0.01 b | 0.04 a | 0.01 b | 0.04 a | 0.04 a | 0.02 ab | 0.01 b | 0.02 ab |
| Palmitic acid (C16:0) | 14.99 b | 14.70 b | 14.18 c | 14.77 b | 15.89 a | 13.40 ef | 13.56 de | 13.07 f | 13.77 d | 13.37 ef | 13.35 ef | 12.64 g | 13.07 f | 9.91 h |
| Palmitoleic acid (C16:1) | 0.99 ef | 1.09 cde | 1.10 cde | 1.10 cde | 1.17 bcd | 1.19 bc | 1.36 a | 1.40 a | 1.23 b | 1.12 bcd | 0.93 f | 1.07 de | 0.92 f | 0.72 g |
| Heptadecanoic acid (C17:0) | 0.04 e | 0.05 de | 0.05 de | 0.05 de | 0.05 de | 0.04 e | 0.14 a | 0.11 b | 0.08 c | 0.06 cde | 0.06 cde | 0.05 de | 0.07 cd | 0.05 de |
| Heptadecenoic acid (C17:1) | 0.07 ef | 0.05 f | 0.09 de | 0.08 de | 0.09 de | 0.08 de | 0.27 a | 0.23 b | 0.16 c | 0.10 d | 0.10 d | 0.10 d | 0.09 de | 0.09 de |
| Stearic acid (C18:0) | 2.81 abc | 3.01 a | 2.39 ef | 2.48 def | 2.28 f | 2.57 cde | 2.48 def | 1.93 g | 2.26 f | 2.28 f | 2.98 ab | 2.48 def | 2.66 cd | 2.72 bcd |
| Oleic acid (C18:1) | 70.30 g | 71.76 f | 71.79 f | 71.12 fg | 71.31 fg | 73.69 d | 73.67 d | 75.09 c | 71.97 ef | 75.41 c | 71.62 f | 76.77 b | 73.18 de | 78.33 a |
| Linoleic acid (C18:2) | 9.25 ab | 7.96 e | 9.04 c | 9.06 bc | 7.72 f | 7.66 f | 6.90 g | 6.13 i | 9.18 bc | 6.28 i | 9.45 a | 5.50 j | 8.56 d | 6.67 h |
| Linolenic acid (C18:3) | 0.72 bc | 0.62 cde | 0.64 cde | 0.65 bcde | 0.71 bc | 0.63 cde | 0.86 a | 0.76 ab | 0.59 def | 0.56 ef | 0.65 bcde | 0.50 f | 0.70 bcd | 0.65 bcde |
| Arachidic acid (C20:0) | 0.43 a | 0.43 a | 0.35 a | 0.36 a | 0.37 a | 0.39 a | 0.37 a | 0.45 a | 0.36 a | 0.38 a | 0.44 a | 0.44 a | 0.37 a | 0.43 a |
| Eicosenoic acid (C20:1) | 0.23 b | 0.19 b | 0.24 b | 0.21 b | 0.25 b | 0.23 b | 0.23 b | 0.56 a | 0.24 b | 0.24 b | 0.23 b | 0.25 b | 0.25 b | 0.26 b |
| Behenic acid (C22:0) | 0.11 abc | 0.09 abc | 0.09 abc | 0.08 bc | 0.10 abc | 0.06 c | 0.11 abc | 0.14 a | 0.11 abc | 0.13 ab | 0.12 ab | 0.13 ab | 0.08 bc | 0.13 ab |
| Lignoceric acid (C24:0) | 0.05 ab | 0.02 b | 0.03 b | 0.02 b | 0.05 ab | 0.03 b | 0.04 ab | 0.09 a | 0.04 ab | 0.03 b | 0.03 b | 0.05 ab | 0.04 ab | 0.02 b |
| Oleic/Linoleic | 7.6 k | 9.0 h | 7.9 j | 7.9 j | 9.2 g | 9.6 f | 10.7 e | 12.3 b | 7.8 j | 12.0 c | 7.6 k | 14.0 a | 8.5 i | 11.7 d |
| ∑SFA | 18.4 ab | 18.3 ab | 17.1 cd | 17.8 bc | 18.8 a | 15.5 de | 16.7 de | 15.8 e | 16.6 de | 16.3 de | 17.0 cd | 15.8 e | 16.3 de | 13.3 f |
| ∑MUFA | 72.0 g | 73.1 ef | 73.2 ef | 72.5 fg | 72.8 fg | 75.2 d | 75.5 cd | 77.3 b | 73.6 ef | 76.9 bc | 72.9 fg | 78.2 ab | 74.4 de | 79.4 a |
| ∑PUFA | 9.9 ab | 8.6 d | 9.7 b | 9.7 b | 8.4 d | 8.3 d | 7.8 e | 6.9 g | 9.8 b | 6.8 g | 10.1 a | 6.0 h | 9.3 c | 7.3 f |
| MUFA/PUFA | 7.2 i | 8.5 f | 7.6 h | 7.5 h | 8.6 f | 9.1 e | 9.7 d | 11.2 b | 7.5 h | 11.2 b | 7.2 i | 13.0 a | 8.0 g | 10.8 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flamminii, F.; Gaggiotti, S.; Chiaudani, A.; Compagnone, D.; Cichelli, A. The Introduction of Allochthonous Olive Variety and Super High-Density System in the Abruzzo Region: A Study on Olive Oil Quality. Foods 2023, 12, 1292. https://doi.org/10.3390/foods12061292
Flamminii F, Gaggiotti S, Chiaudani A, Compagnone D, Cichelli A. The Introduction of Allochthonous Olive Variety and Super High-Density System in the Abruzzo Region: A Study on Olive Oil Quality. Foods. 2023; 12(6):1292. https://doi.org/10.3390/foods12061292
Chicago/Turabian StyleFlamminii, Federica, Sara Gaggiotti, Alessandro Chiaudani, Dario Compagnone, and Angelo Cichelli. 2023. "The Introduction of Allochthonous Olive Variety and Super High-Density System in the Abruzzo Region: A Study on Olive Oil Quality" Foods 12, no. 6: 1292. https://doi.org/10.3390/foods12061292