Hepatoprotective Effect of Albumin Peptide Fractions from Corn Germ Meal against Alcohol-Induced Acute Liver Injury in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
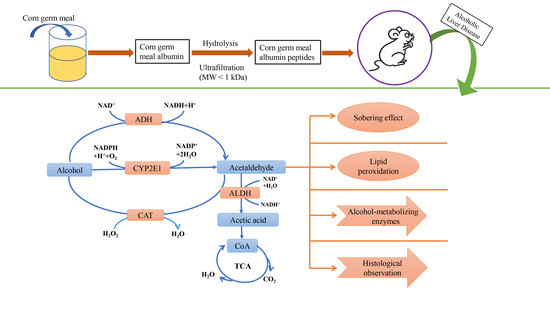
2.2. Preparation of APF4 (MW < 1 kDa)
2.3. Blood Alcohol Concentration (BAC)
2.4. Animal Model to Screen the Maximum Intoxication Dose
2.5. The Sobering Effect of APF4
2.6. The Dose–Response and Time–Response Relationship of APF4 on BAC after Acute Alcohol Intoxication in the Animal Model
2.7. Determination of Liver Biochemical Parameters
2.8. Histopathologic Observation
2.9. Statistical Analysis
3. Results
3.1. The Maximum Intoxication Dose
3.2. The Sobering Effect of APF4
3.3. Effect of APF4 on BAC
3.4. Effect of APF4 on AST, ALT, and TG Levels
3.5. Effect of APF4 on Alcohol-Metabolizing Enzymes, Antioxidant Enzymes, and MDA in the Liver
3.6. Effect of APF4 on Pathological Changes Induced by Alcohol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Bhardwaj, M.; Shukla, S.; Min, S.H.; Choi, D.K.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Carvacrol inhibits cytochrome P450 and protects against binge alcohol-induced liver toxicity. Food Chem. Toxicol. 2019, 131, 110582. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Li, Y.; Yin, S.; Xu, M.; Feng, D.; Zhou, Z.; Zhang, M.; Mukhopadhyay, P.; Varga, Z.V.; Pacher, P.; et al. Aging aggravates alcoholic liver injury and fibrosis in mice by downregulating sirtuin 1 expression. J. Hepatol. 2017, 66, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Jiang, Y.; Zhang, D.; Wang, M.; Chen, W.; Su, H.; Wang, Y.; Wang, J. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, M.J.; Yoon, D.; Lee, Y.S.; Kim, G.S.; Yoo, Y.C. Ginseng Berry Prevents Alcohol-Induced Liver Damage by Improving the Anti-Inflammatory System Damage in Mice and Quality Control of Active Compounds. Int. J. Mol. Sci. 2019, 20, 3522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, B.; Zhang, Y.; Wang, Z.; Ding, Q.; Song, J.; Wang, D. Hepatoprotective Effects of Morchella esculenta against Alcohol-Induced Acute Liver Injury in the C57BL/6 Mouse Related to Nrf-2 and NF-kappa B Signaling. Oxidative Med. Cell. Longev. 2019, 2019, 6029876. [Google Scholar] [CrossRef] [Green Version]
- Zhong, F.; Hu, Z.; Jiang, K.; Lei, B.; Wu, Z.; Yuan, G.; Luo, H.; Dong, C.; Tang, B.; Zheng, C.; et al. Complement C3 activation regulates the production of tRNA-derived fragments Gly-tRFs and promotes alcohol-induced liver injury and steatosis. Cell Res. 2019, 29, 548–561. [Google Scholar] [CrossRef] [Green Version]
- She, X.; Wang, F.; Ma, J.; Chen, X.; Ren, D.; Lu, J. In vitro antioxidant and protective effects of corn peptides on ethanol-induced damage in HepG2 cells. Food Agric. Immunol. 2016, 27, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Li, J.; He, H.; Huang, W.; Zhang, W. Ultrafiltration preparation of potent bioactive corn peptide as alcohol metabolism stimulator in vivo and study on its mechanism of action. J. Food Biochem. 2013, 37, 161–167. [Google Scholar] [CrossRef]
- Qu, L.; Zhu, Y.; Liu, Y.; Yang, H.; Zhu, C.; Ma, P.; Deng, J.; Fan, D. Protective effects of ginsenoside Rk3 against chronic alcohol-induced liver injury in mice through inhibition of inflammation, oxidative stress, and apoptosis. Food Chem. Toxicol. 2019, 126, 277–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, F.; Li, C.; Li, W.; Li, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X. White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation. Antioxidants 2019, 8, 524. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Yang, Y.; Cao, X.; Zhao, Y.; Gao, X.; Sun, C.; Zhang, F.; Yuan, Y.; Xu, Y.; Zhang, J.; et al. Plin3 protects against alcoholic liver injury by facilitating lipid export from the endoplasmic reticulum. J. Cell. Biochem. 2019, 120, 16075–16087. [Google Scholar] [CrossRef]
- Feng, L.; Chen, J.; Yan, W.; Ye, Z.; Yu, J.; Yao, G.; Wu, Y.; Zhang, J.; Yang, D. Preparation of Active Peptides from Camellia vietnamensis and Their Metabolic Effects in Alcohol-Induced Liver Injury Cells. Molecules 2022, 27, 1790. [Google Scholar] [CrossRef]
- Cho, H.R.; Lee, S.O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef]
- Ren, J.; Sha, W.; Shang, S.; Yuan, E. Hepatoprotective peptides purified from Corbicula fluminea and its effect against ethanol-induced LO2 cells injury. Int. J. Food Sci. Technol. 2021, 56, 352–361. [Google Scholar] [CrossRef]
- Ren, J.; Li, S.; Song, C.; Sun, X.; Liu, X. Black soybean-derived peptides exerted protective effect against alcohol-induced liver injury in mice. J. Funct. Foods 2021, 87, 104828. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Xing, R.; Li, P. Hepatoprotective Effect of Oyster Peptide on Alcohol-Induced Liver Disease in Mice. Int. J. Mol. Sci. 2022, 23, 8081. [Google Scholar] [CrossRef]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.S.; Ahn, C.B.; Ahn, G. In Vivo Hepatoprotective Effects of a Peptide Fraction from Krill Protein Hydrolysates against Alcohol-Induced Oxidative Damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, L.; Wang, Y.; Lin, D.; Liu, J. Hepatoprotective Effect of Albumin Peptides from Corn Germ Meal on Chronic Alcohol-Induced Liver Injury in Mice. J. Food Sci. 2017, 82, 2997–3004. [Google Scholar] [CrossRef]
- Liang, J.; Li, Q.; Lin, B.; Yu, Y.; Ding, Y.; Dai, X.; Li, Y. Comparative studies of oral administration of marine collagen peptides from Chum Salmon (Oncorhynchus keta) pre- and post-acute ethanol intoxication in female Sprague-Dawley rats. Food Funct. 2014, 5, 2078–2085. [Google Scholar] [CrossRef]
- Harrison, N.L.; Skelly, M.J.; Grosserode, E.K.; Lowes, D.C.; Zeric, T.; Phister, S.; Sailing, M.C. Effects of acute alcohol on excitability in the CNS. Neuropharmacology 2017, 122, 36–45. [Google Scholar] [CrossRef]
- Diao, X.; Zhou, Z.; Xiang, W.; Jiang, Y.; Tian, N.; Tang, X.; Chen, S.; Wen, J.; Chen, M.; Liu, K.; et al. Glutathione alleviates acute intracerebral hemorrhage injury via reversing mitochondrial dysfunction. Brain Res. 2020, 1727, 146514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Manna, S.K.; Golla, S.; Krausz, K.W.; Cai, Y.; Garcia-Milian, R.; Chakraborty, T.; Chakraborty, J.; Chatterjee, R.; Thompson, D.C.; et al. Glutathione deficiency-elicited reprogramming of hepatic metabolism protects against alcohol-induced steatosis. Free Radic. Biol. Med. 2019, 143, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, F.; Zhao, M.; Su, G.; Sun, B. Chicken breast muscle hydrolysates ameliorate acute alcohol-induced liver injury in mice through alcohol dehydrogenase (ADH) activation and oxidative stress reduction. Food Funct. 2018, 9, 774–784. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, P.; Xu, H.; Niu, J.; Gao, Y. Growing burden of alcoholic liver disease in China. World J. Gastroenterol. 2019, 25, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sha, Y.; Wang, B. Relationship between alcohol consumption and the risks of liver cancer, esophageal cancer, and gastric cancer in China Meta-analysis based on case-control studies. Medicine 2021, 100, e26982. [Google Scholar] [CrossRef]
- Bukong, T.N.; Iracheta-Vellve, A.; Gyongyosi, B.; Ambade, A.; Catalano, D.; Kodys, K.; Szabo, G. Therapeutic Benefits of Spleen Tyrosine Kinase Inhibitor Administration on Binge Drinking-Induced Alcoholic Liver Injury, Steatosis, and Inflammation in Mice. Alcoholism 2016, 40, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Zhang, F.; Yu, Y.; Jiang, Q.; Zhang, Z.; Wang, J.; Li, Y. Marine collagen peptides protect against early alcoholic liver injury in rats. Br. J. Nutr. 2012, 107, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, C.; Qin, X.; Cao, W.; Chen, J.; Li, Y.; Zheng, H.; Lin, H.; Chen, Z. Hepatoprotective effect of clam (Corbicula fluminea) protein hydrolysate on alcohol-induced liver injury in mice and partial identification of a hepatoprotective peptide from the hydrolysate. Food Sci. Technol. 2022, 42, e61522. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Qu, Y. Antagonistic effect of the glycopeptide from zein on acute alcohol-induced liver injury in mice. J. Funct. Foods 2022, 92, 105062. [Google Scholar] [CrossRef]
- Pan, F.; Cai, Z.; Ge, H.; Ma, S.; Yu, Y.; Liu, J.; Zhang, T. Transcriptome analysis reveals the hepatoprotective mechanism of soybean meal peptides against alcohol-induced acute liver injury mice. Food Chem. Toxicol. 2021, 154, 112353. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Zi, Y.; Zhao, H.; Wang, H.; Zhang, Y. Functional coix seed protein hydrolysates as a novel agent with potential hepatoprotective effect. Food Funct. 2020, 11, 9495–9502. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol Metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Fang, H.; Lin, W. Post-Treatment of Ganoderma lucidum Reduced Liver Fibrosis Induced by Thioacetamide in Mice. Phytother. Res. 2010, 24, 494–499. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Guo, H.; Sun, J.; He, H.; Yu, G.; Du, J. Antihepatotoxic effect of corn peptides against Bacillus Calmette-Guerin/lipopolysaccharide-induced liver injury in mice. Food Chem. Toxicol. 2009, 47, 2431–2435. [Google Scholar] [CrossRef]
- Koop, D.R. Alcohol metabolism’s damaging effects on the cell—A focus on reactive oxygen generation by the enzyme cytochrome P450 2E1. Alcohol Res. Health 2006, 29, 274–280. [Google Scholar]
- Sen Raychaudhuri, S.; Deng, X. The role of superoxide dismutase in combating oxidative stress in higher plants. Bot. Rev. 2000, 66, 89–98. [Google Scholar] [CrossRef]




| Alcohol Intake (mL/kg/bw) | Number | Intoxication Rate (%) | Mortality Rate (%) |
|---|---|---|---|
| 15 | 10 | 100 | 50 |
| 14 | 10 | 90 | 40 |
| 13 | 10 | 90 | 10 |
| 12 | 10 | 70 | 0 |
| 11 | 10 | 20 | 0 |
| Groups | Materials | Intoxication Rate (%) | Mortality Rate (%) | Loss of Righting Reflex (min) | Recovery Time of Righting Reflex (min) |
|---|---|---|---|---|---|
| Model | Saline + Alcohol | 80 | 0 | 12.00 ± 4.60 | 171.38 ± 47.05 |
| Positive | GSH + Alcohol | 70 | 0 | 24.50 ± 9.85 * | 98.00 ± 28.92 ** |
| APF4-200 | APF4 + Alcohol | 70 | 0 | 18.29 ± 8.96 | 143.71 ± 53.15 |
| APF4-400 | APF4 + Alcohol | 60 | 0 | 26.17 ± 10.55 ** | 98.33 ± 40.88 ** |
| APF4-800 | APF4 + Alcohol | 60 | 0 | 32.67 ± 11.71 ** | 61.67 ± 19.09 ** |
| BAC (mg/mL) | |||||
|---|---|---|---|---|---|
| Time (h) | Model | Positive | APF4-200 | APF4-400 | APF4-800 |
| 0.5 | 2.88 ± 0.26 | 2.58 ± 0.25 * | 2.68 ± 0.42 | 2.09 ± 0.38 ** | 2.37 ± 0.15 ** |
| 1.0 | 4.20 ± 0.30 | 3.92 ± 0.15 * | 3.99 ± 0.31 | 3.44 ± 0.31 ** | 2.71 ± 0.50 ** |
| 1.5 | 4.72 ± 0.35 | 4.50 ± 0.33 | 4.37 ± 0.37 * | 4.10 ± 0.27 ** | 3.64 ± 0.26 ** |
| 2.0 | 4.43 ± 0.40 | 4.33 ± 0.20 | 4.23 ± 0.35 | 3.90 ± 0.17 ** | 3.10 ± 0.25 ** |
| 3.0 | 3.39 ± 0.29 | 2.62 ± 0.24 ** | 3.04 ± 0.15 ** | 2.29 ± 0.27 ** | 2.21 ± 0.26 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Guan, S.; Feng, M.; Wang, L.; Gao, F. Hepatoprotective Effect of Albumin Peptide Fractions from Corn Germ Meal against Alcohol-Induced Acute Liver Injury in Mice. Foods 2023, 12, 1183. https://doi.org/10.3390/foods12061183
Yu Y, Guan S, Feng M, Wang L, Gao F. Hepatoprotective Effect of Albumin Peptide Fractions from Corn Germ Meal against Alcohol-Induced Acute Liver Injury in Mice. Foods. 2023; 12(6):1183. https://doi.org/10.3390/foods12061183
Chicago/Turabian StyleYu, Yali, Shiyao Guan, Mengmeng Feng, Lijun Wang, and Feng Gao. 2023. "Hepatoprotective Effect of Albumin Peptide Fractions from Corn Germ Meal against Alcohol-Induced Acute Liver Injury in Mice" Foods 12, no. 6: 1183. https://doi.org/10.3390/foods12061183