Mitigation of Salmonella on Food Contact Surfaces by Using Organic Acid Mixtures Containing 2-Hydroxy-4-(methylthio) Butanoic Acid (HMTBa)
Abstract
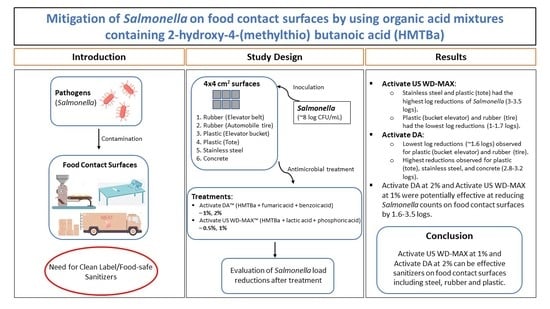
:1. Introduction
2. Materials and Methods
2.1. Organic Acid Mixtures
2.2. Inoculum Preparation
2.3. Food Contact Surface Materials and Inoculation
2.4. Confirmative Test for Salmonella
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heredia, N.; Garcia, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Eng, S.K.; Pusparajah, P.; Ab Mutalib, N.S.; Ser, H.L.; Chan, K.G.; Lee, L.H. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Life Sci. 2015, 8, 284–293. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration (FDA). Information on Marketing a Pet Food Product. Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/information-marketing-pet-food-product/ (accessed on 9 January 2023).
- U.S. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/ (accessed on 12 July 2021).
- Food and Drug Administration (FDA). Human Food By-Products for Use as Animal Food. Draft Guidance for Industry #239. 2016. Available online: https://www.fda.gov/downloads/AnimalVeterinary/ (accessed on 4 June 2021).
- Scott, E.; Bloomfield, S.F. The survival and transfer of microbial contamination via cloths, hands and utensils. J. Appl. Bacteriol. 1990, 68, 271–278. [Google Scholar] [CrossRef]
- Dunsmore, D.G.; Twomey, A.; Whittlestone, W.G.; Morgan, H.W. Design and performance of systems for cleaning product-contact surfaces of food equipment: A review. J. Food Prot. 1981, 44, 220–240. [Google Scholar] [CrossRef]
- Schumacher, L.L.; Cochrane, R.A.; Huss, A.R.; Gebhardt, J.T.; Woodworth, J.C.; Stark, C.R.; Jones, C.K.; Bai, J.; Main, R.G.; Chen, Q.; et al. Feed batch sequencing to decrease the risk of porcine epidemic diarrhea virus (PEDV) cross-contamination during feed manufacturing. J. Anim. Sci. 2018, 96, 4562–4570. [Google Scholar] [CrossRef]
- Huss, A.R.; Cochrane, R.A.; Deliephan, A.; Stark, C.R.; Jones, C.K. Evaluation of a biological pathogen decontamination protocol for animal feed mills. J. Food Prot. 2015, 78, 1682–1688. [Google Scholar] [CrossRef]
- Habimana, O.; Moretro, T.; Langsrud, S.; Vestby, L.K.; Nesse, L.L.; Heir, E. Micro ecosystems from feed industry surfaces: A survival and biofilm study of Salmonella versus host resident flora strains. BMC Vet. Res. 2010, 6, 48. [Google Scholar] [CrossRef]
- Jullien, C.; Benezech, T.; Carpentier, B.; Lebret, V.; Faille, C. Identification of surface characteristics relevant to the hygienic status of stainless steel for the food industry. J. Food Eng. 2003, 56, 77–87. [Google Scholar] [CrossRef]
- Schumacher, L.L.; Cochrane, R.A.; Evans, C.E.; Kalivoda, J.R.; Woodworth, J.C.; Huss, A.R.; Stark, C.R.; Jones, C.K.; Chen, Q.; Main, R.; et al. Evaluating the effect of manufacturing porcine epidemic diarrhea virus (PEDV)-contaminated feed on subsequent feed mill environmental surface contamination. J. Anim. Sci. 2016, 94, 77. [Google Scholar] [CrossRef] [Green Version]
- Kaewtapee, C.; Krutthai, N.; Poosuwan, K.; Poeikhampha, T.; Koonawootrittriron, S.; Bunchasak, C. Effects of adding liquid dl-methionine hydroxy analogue-free acid to drinking water on growth performance and small intestinal morphology of nursery pigs. J. Anim. Physiol. Anim. Nutr. 2010, 94, 395–404. [Google Scholar] [CrossRef]
- Swennen, Q.; Geraert, P.A.; Mercier, Y.; Everaert, N.; Stinckens, A.; Willemsen, H.; Li, Y.; Decuypere, E.; Buyse, J. Effects of dietary protein content and 2-hydroxy-4-methylthiobutanoic acid or DL-methionine supplementation on performance and oxidative status of broiler chickens. Br. J. Nutr. 2011, 106, 1845–1854. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Patterson, P.H.; Kim, W.K. Impact of dietary crude protein, synthetic amino acid and keto acid formulation on nitrogen excretion. Int. J. Poult. Sci. 2014, 13, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.D.; Dibner, J.J. Comparative absorption of 2-hydroxy-4-(methylthio)-butanoic acid and L-methionine in the broiler chick. J. Nutr. 1984, 114, 2179–2186. [Google Scholar] [CrossRef]
- Dibner, J.J.; Atwell, C.A.; Ivey, F.J. Effect of heat stress on 2-hydroxy-4-(methylthio) butanoic acid and DL-methionine absorption measured in vitro. Poult. Sci. 1992, 71, 1900–1910. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, W.W.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.D.; Li, S.H.; Zhou, X.Q. Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2011, 17, 595–604. [Google Scholar] [CrossRef]
- Willemsen, H.; Swennen, Q.; Everaert, N.; Geraert, P.A.; Mercier, Y.; Stinckens, A.; Decuypere, E.; Buyse, J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011, 90, 2311–2320. [Google Scholar] [CrossRef]
- Kuang, S.Y.; Xiao, W.W.; Feng, L.; Liu, Y.; Jiang, J.; Jiang, W.D.; Hu, K.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var. Jian). Fish. Shellfish. Immunol 2012, 32, 629–636. [Google Scholar] [CrossRef]
- Li, H.; Wan, H.; Mercier, Y.; Zhang, X.; Wu, C.; Wu, X.; Tang, L.; Che, L.; Lin, Y.; Xu, S.; et al. Changes in plasma amino acid profiles, growth performance and intestinal antioxidant capacity of piglets following increased consumption of methionine as its hydroxy analogue. Br. J. Nutr. 2014, 112, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Quiroz, M. Water Quality and Broiler Performance. North Carolina Broiler Supervisor’s Short Course, April 17, 2008. Available online: https://www.researchgate.net/profile/Edgar-Oviedo-3/publication/242376063 (accessed on 4 April 2021).
- Deliephan, A. Reducing Spoilage in Intermediate Moisture Pet Foods Using Food-Safe Additives as a Model System. Ph.D. Dissertation, Kansas State University, Manhattan, KS, USA, 2022. [Google Scholar]
- Kim, H.J.; Park, S.H.; Lee, T.H.; Nahm, B.H.; Chung, Y.H.; Seo, K.H.; Kim, H.Y. Identification of Salmonella enterica serovar Typhimurium using specific PCR primers obtained by comparative genomics in Salmonella serovars. J. Food Prot. 2006, 69, 1653–1661. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Muckey, M.B. Evaluation of Surface Sanitation to Prevent Biological Hazards in Animal Food Manufacturing. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2016. [Google Scholar]
- Okelo, P.O.; Wagner, D.D.; Carr, L.E.; Wheaton, F.W.; Douglass, L.W.; Joseph, S.W. Optimization of extrusion conditions for elimination of mesophilic bacteria during thermal processing of animal feed mash. Anim. Feed. Sci. Technol. 2006, 129, 116–137. [Google Scholar] [CrossRef]
- Cochrane, R.A.; Schumacher, L.L.; Dritz, S.S.; Woodworth, J.C.; Huss, A.R.; Stark, C.R.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Bai, J.; et al. Effect of thermal mitigation on porcine epidemic diarrhea virus (PEDV)-contaminated feed. Kans Agric. Exp. Stn Res. Rep. 2015, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Jones, F.T. A review of practical Salmonella control measures in animal feed. J. Appl Poult Res. 2011, 20, 102–113. [Google Scholar] [CrossRef]
- FAO, I. Good practices for the feed industry-Implementing the codex alimentarius code of practice on good animal feeding. FAO Anim. Prod. Health Man 2010, 9, 79. [Google Scholar]
- Davies, R.H.; Wales, A.D. Investigations into Salmonella contamination in poultry feed mills in the United Kingdom. J. Appl. Microbiol. 2010, 109, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Ronner, A.B.; Wong, A.C. Biofilm development and sanitizer inactivation of Listeria monocytogenes and Salmonella Typhimurium on stainless steel and Buna-n rubber. J. Food Prot. 1993, 56, 750–758. [Google Scholar] [CrossRef]
- Shen, C.; Luo, Y.; Nou, X.; Bauchan, G.; Zhou, B.; Wang, Q.; Millner, P. Enhanced inactivation of Salmonella and Pseudomonas biofilms on stainless steel by use of T-128, a fresh-produce washing aid, in chlorinated wash solutions. Appl. Environ. Microbiol. 2012, 78, 6789–6798. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.A.; Oladunjoye, A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L.; Mikel, B.; Bailey, R.H. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless-steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Prot. 2013, 76, 205–212. [Google Scholar] [CrossRef]
- Desai, M.A.; Soni, K.A.; Nannapaneni, R.; Schilling, M.W.; Silva, J.L. Reduction of Listeria monocytogenes biofilms on stainless steel and polystyrene surfaces by essential oils. J. Food Prot. 2012, 75, 1332–1337. [Google Scholar] [CrossRef]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef]
- Kashket, E.R. Bioenergetics of lactic acid bacteria: Cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987, 46, 233–244. [Google Scholar] [CrossRef]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci Prog 2003, 86, 245–269. [Google Scholar] [CrossRef]
- Salsali, H.R.; Parker, W.J.; Sattar, S.A. The effect of volatile fatty acids on the inactivation of Clostridium perfingens in anaerobic digestion. World J. Microbiol. Biotechnol. 2008, 24, 659–665. [Google Scholar] [CrossRef]
- Marriott, N.G.; Gravani, R.B.; Schilling, M.W. Principles of Food Sanitation; Springer Publishing: New York, NY, USA, 2006; Volume 22. [Google Scholar]



| Food Contact Surface | Salmonella log Reduction (log CFU/cm2) (Mean ± SE) 1,2,3 | ||||
|---|---|---|---|---|---|
| Untreated | Activate US WD-MAX | Activate DA | |||
| 0.0% | 0.5% | 1.0% | 1.0% | 2.0% | |
| Stainless steel | 0.4 ± 0.1 a,B | 3.0 ± 0.4 b,B | 3.5 ± 0.4 b,B | 2.4 ± 0.2 b,B | 3.2 ± 0.1 b,B |
| Plastic (Bucket elevator) | 0.5 ± 0.1 a,D | 1.0 ± 0.3 b,D | 1.7 ± 0.3 b,D | 1.0 ± 0.3 b,D | 1.6 ± 0.1 b,D |
| Plastic (Tote) | 2.5 ± 0.4 a,A | 2.9 ± 0.2 b,A | 3.3 ± 0.4 b,A | 2.8 ± 0.3 b,A | 3.2 ± 0.4 b,A |
| Concrete | 2.3 ± 0.2 a,AB | 2.9 ± 0.1 b,AB | 3.0 ± 0.2 b,AB | 2.6 ± 0.4 b,AB | 2.8 ± 0.2 b,AB |
| Rubber (Belt) | 2.0 ± 0.2 a,AB | 2.8 ± 0.1 b,AB | 2.9 ± 0.1 b,AB | 2.5 ± 0.4 b,AB | 2.8 ± 0.2 b,AB |
| Rubber (Tire) | 1.6 ± 0.4 a,C | 1.9 ± 0.3 b,C | 1.8 ± 0.2 b,C | 1.8 ± 0.2 b,C | 1.7 ± 0.4 b,C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deliephan, A.; Dhakal, J.; Subramanyam, B.; Aldrich, C.G. Mitigation of Salmonella on Food Contact Surfaces by Using Organic Acid Mixtures Containing 2-Hydroxy-4-(methylthio) Butanoic Acid (HMTBa). Foods 2023, 12, 874. https://doi.org/10.3390/foods12040874
Deliephan A, Dhakal J, Subramanyam B, Aldrich CG. Mitigation of Salmonella on Food Contact Surfaces by Using Organic Acid Mixtures Containing 2-Hydroxy-4-(methylthio) Butanoic Acid (HMTBa). Foods. 2023; 12(4):874. https://doi.org/10.3390/foods12040874
Chicago/Turabian StyleDeliephan, Aiswariya, Janak Dhakal, Bhadriraju Subramanyam, and Charles G. Aldrich. 2023. "Mitigation of Salmonella on Food Contact Surfaces by Using Organic Acid Mixtures Containing 2-Hydroxy-4-(methylthio) Butanoic Acid (HMTBa)" Foods 12, no. 4: 874. https://doi.org/10.3390/foods12040874