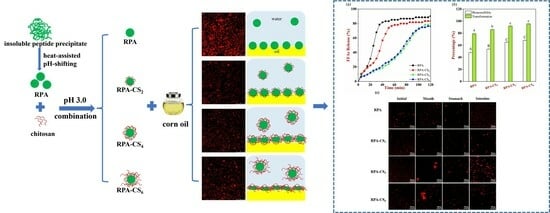
Properties of Heat-Assisted pH Shifting and Compounded Chitosan from Insoluble Rice Peptide Precipitate and Its Application in the Curcumin-Loaded Pickering Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Insoluble Peptide Precipitate and Rice Peptide Aggregate (RPA)
2.3. Preparation of Rice Peptide Aggregate–Chitosan (RPA–CS) Complexes
2.4. ζ-Potential, Turbidity, and Z-Average Diameter of RPA–CS Complexes
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Scanning Electron Microscopy (SEM)
2.7. Interfacial Properties
2.7.1. Wettability Measurement
2.7.2. Dynamic Interfacial Tension
2.7.3. Quartz Crystal Microbalance with Dissipation (QCM–D) Experiments
2.8. Fabrication of Curcumin–Loaded Pickering Emulsions
2.9. Storage Stability of Curcumin–Loaded Emulsions
2.10. Microrheological Properties of Curcumin-Loaded Emulsions
2.11. In Vitro Digestion
2.12. Statistical Analysis
3. Results and Discussion
3.1. Effect of pH on the RPA–CS Complexes
3.1.1. Turbidity
3.1.2. ζ–Potential Analysis
3.2. Fourier Infrared Spectroscopy
3.3. Characterization of RPA–CS Complexes
3.4. Interfacial Properties
3.4.1. Wettability Analysis of RPA–CS Complexes
3.4.2. Interfacial Tension Analysis of RPA–CS Complexes
3.4.3. Adsorption of RPA–CS Complexes on Hydrophobic Surfaces
3.5. Storage Stability Analysis of Emulsions
3.6. Microrheological Properties
3.7. In Vitro Simulation of Changes in Emulsions during Digestion
3.7.1. Initial System
3.7.2. Mouth Phase
3.7.3. Stomach Phase
3.7.4. Small Intestine Phase
3.8. Lipid Digestion Profiles
3.9. Curcumin Bioaccessibility and Transformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Liu, W.; Liu, C.; Luo, L.; Chen, J.; Luo, S.; McClements, D.J.; Wu, L. Effect of limited enzymatic hydrolysis on structure and emulsifying properties of rice glutelin. Food Hydrocoll. 2016, 61, 251–260. [Google Scholar] [CrossRef]
- Marcet, I.; Alvarez, C.; Paredes, B.; Diaz, M. Inert and oxidative subcritical water hydrolysis of insoluble egg yolk granular protein, functional properties, and comparison to enzymatic hydrolysis. J. Agric. Food Chem. 2014, 62, 8179–8186. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, F.; Kong, Z.; Yang, Z.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; Xu, X. Pickering emulsion gel stabilized by pea protein nanoparticle induced by heat-assisted pH-shifting for curcumin delivery. J. Food Eng. 2023, 350, 111504. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, Y.; Wen, H.; Zhang, Y.; Liu, J.; Zhang, T. Mild heating assisted alkaline pH shifting modify the egg white protein: The mechanism and the enhancement of emulsifying properties. LWT-Food Sci. Technol. 2021, 151, 112094. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G. Fabricating a pickering stabilizer from Okara dietary fibre particulates by conjugating with soy protein isolate via Maillard reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; McClements, D.J. Encapsulation of vitamin D3 in pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, W. Micro/nano emulsion delivery systems: Effects of potato protein/chitosan complex on the stability, oxidizability, digestibility and β—Carotene release characteristics of the emulsion. Innov. Food Sci. Emerg. Technol. 2022, 77, 102980. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, L.; Liu, Y.; Li, J. Mayonnaise-like high internal phase Pickering emulsions stabilized by co-assembled phosphorylated perilla protein isolate and chitosan for extrusion 3D printing application. Food Hydrocoll. 2023, 135, 108133. [Google Scholar] [CrossRef]
- Xu, X.; Luo, L.; Liu, C.; Zhang, Z.; McClements, D.J. Influence of electrostatic interactions on behavior of mixed rice glutelin and alginate systems: pH and ionic strength effects. Food Hydrocoll. 2017, 63, 301–308. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Boostani, S.; Hosseini, S.M.H.; Yousefi, G.; Riazi, M.; Tamaddon, A.-M.; Van der Meeren, P. The stability of triphasic oil-in-water Pickering emulsions can be improved by physical modification of hordein- and secalin-based submicron particles. Food Hydrocoll. 2019, 89, 649–660. [Google Scholar] [CrossRef]
- Zhou, B.; Tobin, J.T.; Drusch, S.; Hogan, S.A. Dynamic adsorption and interfacial rheology of whey protein isolate at oil-water interfaces: Effects of protein concentration, pH and heat treatment. Food Hydrocoll. 2021, 116, 10640. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, L.; Sun, Q.; McClements, D.J.; Xu, X. Effect of molecular weight on the interfacial and emulsifying characteristics of rice glutelin hydrolysates. Food Hydrocoll. 2022, 128, 107560. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Su, C.; Wu, J.; Van der Meeren, P. Effect of ionic strength on the sequential adsorption of whey proteins and low methoxy pectin on a hydrophobic surface: A QCM-D study. Food Hydrocoll. 2022, 122, 107074. [Google Scholar] [CrossRef]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Bahmaei, M. Characterization of sodium caseinate/Hydroxypropyl methylcellulose concentrated emulsions: Effect of mixing ratio, concentration and wax addition. Int. J. Biol. Macromol. 2019, 128, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Fazelioskouei, T.; Tabibiazar, M.; Mohammadifar, M.; Masoumi, B.; Homayouni-rad, A. Fabrication and characterization of food-grade pea protein-ascorbic acid nanoparticles-stabilized Pickering emulsion. J. Mol. Liq. 2023, 388, 122829. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Hu, T.; Xu, X.; Pan, S.; Hu, H. Effects of different ionic strengths on the physicochemical properties of plant and animal proteins-stabilized emulsions fabricated using ultrasound emulsification. Ultrason Sonochem. 2019, 58, 104627. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Duan, Y.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; Xu, X. Hydrolyzed rice glutelin nanoparticles as particulate emulsifier for Pickering emulsion: Structure, interfacial properties, and application for encapsulating curcumin. Food Hydrocoll. 2023, 134, 108105. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.S.; Sarkar, A. Overcoming in vitro gastric destabilisation of emulsion droplets using emulsion microgel particles for targeted intestinal release of fatty acids. Food Hydrocoll. 2019, 89, 523–533. [Google Scholar] [CrossRef]
- Yucetepe, A.; Yavuz-Duzgun, M.; Sensu, E.; Bildik, F.; Demircan, E.; Ozcelik, B. The impact of pH and biopolymer ratio on the complex coacervation of Spirulina platensis protein concentrate with chitosan. J. Food Sci. Technol. 2021, 58, 1274–1285. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Selective complex coacervation of pea whey proteins with chitosan to purify main 2S albumins. J. Agric. Food Chem. 2020, 68, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhao, J.; Jiang, Z.; Mu, J.; Huang, L.; Dai, C. Fabrication of whey protein isolate/chitosan complexes and its protective effect on allicin. J. Appl. Polym. 2022, 140, e53579. [Google Scholar] [CrossRef]
- Xie, H.; Ouyang, K.; Shi, W.; Wang, W.; Wang, Y.; Xiong, H.; Zhao, Q. Enhancing the interfacial stability of O/W emulsion by adjusting interactions of chitosan and rice protein hydrolysate. Food Hydrocoll. 2023, 137, 108406. [Google Scholar] [CrossRef]
- Figueroa-González, J.J.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Aguirre-Mandujano, E.; Alvarez-Ramirez, J.; Martínez-Velasco, A. Modifying the structure, physicochemical properties, and foaming ability of amaranth protein by dual pH-shifting and ultrasound treatments. LWT-Food Sci. Technol. 2022, 153, 112561. [Google Scholar] [CrossRef]
- Tavares, L.; Souza, H.K.S.; Gonçalves, M.P.; Rocha, C.M.R. Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate. Food Hydrocoll. 2021, 113, 106471. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, X.; Jiang, Y.; Ban, Q.; Wang, X. Enhancing the stability of oil-in-water emulsions by non-covalent interaction between whey protein isolate and hyaluronic acid. Int. J. Biol. Macromol. 2023, 225, 1085–1095. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, J. Extrusion-based 3D printing of high internal phase emulsions stabilized by co-assembled β-cyclodextrin and chitosan. Food Hydrocoll. 2023, 134, 108036. [Google Scholar] [CrossRef]
- Lopes, I.S.; Michelon, M.; Duarte, L.G.R.; Prediger, P.; Cunha, R.L.; Picone, C.S.F. Effect of chitosan structure modification and complexation to whey protein isolate on oil/water interface stabilization. Chem. Eng. Sci. 2021, 230, 116124. [Google Scholar] [CrossRef]
- Qiao, X.; Jiang, Y.; Duan, R.; Li, Z.; Kong, Z.; Zhang, L.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; et al. Fabrication of curcumin-loaded pea protein isolate-quillaja saponin-tannic acid self-assembled nanoparticles by tuning non-covalent interactions: Enhanced physicochemical, interfacial and emulsifying properties. Food Hydrocoll. 2024, 148, 109436. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Zhang, S.; Dongye, Z.; Kang, M.; Li, Z.; Chen, C.; Qian, Y.; Ren, Y. The rheological/interfacial behavior and stability properties of nanoemulsions prepared using whey protein-carboxymethyl chitosan conjugates. Colloid Surf. A 2023, 662, 130924. [Google Scholar] [CrossRef]
- Niu, H.; Chen, X.; Luo, T.; Chen, H.; Fu, X. The interfacial behavior and long-term stability of emulsions stabilized by gum arabic and sugar beet pectin. Carbohydr. Polym. 2022, 291, 119623. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, Q.; Yang, S.; Li, H.; Mao, L.; Gao, Y.; Yuan, F. Electrostatic deposition of polysaccharide onto soft protein colloidal particles: Enhanced rigidity and potential application as Pickering emulsifiers. Food Hydrocoll. 2021, 110, 106147. [Google Scholar] [CrossRef]
- Du, Q.; Wu, Y.; Zeng, X.; Tu, M.; Wu, Z.; Liu, J.; Pan, D.; Ding, Y. Competitive binding of maltodextrin and pectin at the interface of whey protein hydrolyzate-based fish oil emulsion under high temperature sterilization: Effects on storage stability and in vitro digestion. Food Res. Int. 2023, 164, 112368. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wu, Y.; Zhong, L.; Ma, N.; Zhao, L.; Ma, G.; Cheng, N.; Nakata, P.A.; Xu, J. In vitro digestion and cellular antioxidant activity of β-carotene-loaded emulsion stabilized by soy protein isolate-Pleurotus eryngii polysaccharide conjugates. Food Hydrocoll. 2021, 112, 106340. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Q.; McClements, D.J. Effects of anionic polysaccharides on the digestion of fish oil-in-water emulsions stabilized by hydrolyzed rice glutelin. Food Res. Int. 2020, 127, 108768. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-water Pickering emulsions via microfluidization with cellulose nanocrystals: 2. In vitro lipid digestion. Food Hydrocoll. 2019, 96, 709–716. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Salt, L.J.; Ridout, M.J.; Ding, Y.; Wilde, P.J. Fish oil emulsions stabilized with caseinate glycated by dextran: Physicochemicalstability and gastrointestinal fate. J. Agric. Food Chem. 2019, 67, 452–462. [Google Scholar] [CrossRef]









| Sample | K1 × 106 (Hz−1) | K2 × 106 (Hz−1) | K3 × 106 (Hz−1) | K4 × 106 (Hz−1) |
|---|---|---|---|---|
| RPA | 0.112 ± 0.038 a | 0.122 ± 0.001 a | 0.299 ± 0.037 a | 0.084 ± 0.001 a |
| RPA–CS2 | 0.331 ± 0.05 b | 0.104 ± 0.095 a | 0.667 ± 0.004 b | 0.143 ± 0.047 ab |
| RPA–CS4 | 0.451 ± 0.004 c | 0.459 ± 0.084 b | 0.715 ± 0.061 b | 0.170 ± 0.013 b |
| RPA–CS6 | 0.592 ± 0.018 d | 0.659 ± 0.002 c | 0.677 ± 0.001 b | 0.487 ± 0.026 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Li, Z.; Xu, Z.; Kong, Z.; Qiao, X.; Zhang, L.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; et al. Properties of Heat-Assisted pH Shifting and Compounded Chitosan from Insoluble Rice Peptide Precipitate and Its Application in the Curcumin-Loaded Pickering Emulsions. Foods 2023, 12, 4384. https://doi.org/10.3390/foods12244384
Yang Z, Li Z, Xu Z, Kong Z, Qiao X, Zhang L, Dai L, Wang Y, Sun Q, McClements DJ, et al. Properties of Heat-Assisted pH Shifting and Compounded Chitosan from Insoluble Rice Peptide Precipitate and Its Application in the Curcumin-Loaded Pickering Emulsions. Foods. 2023; 12(24):4384. https://doi.org/10.3390/foods12244384
Chicago/Turabian StyleYang, Zhenyu, Zhiying Li, Zitong Xu, Zhihao Kong, Xin Qiao, Liwen Zhang, Lei Dai, Yanfei Wang, Qingjie Sun, David Julian McClements, and et al. 2023. "Properties of Heat-Assisted pH Shifting and Compounded Chitosan from Insoluble Rice Peptide Precipitate and Its Application in the Curcumin-Loaded Pickering Emulsions" Foods 12, no. 24: 4384. https://doi.org/10.3390/foods12244384