Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives
Abstract
:1. Introduction
2. Microstructure and Macrostructure Formation during HME
2.1. Mixing of Dry and Wet Feed
2.2. Liquefaction and Aggregation
2.3. Protein Alignment and Phase Separation
2.4. Phase Elongation (Protein, Fat Droplets, and Gas)
2.5. Cross-Linking, Solidification, and Syneresis
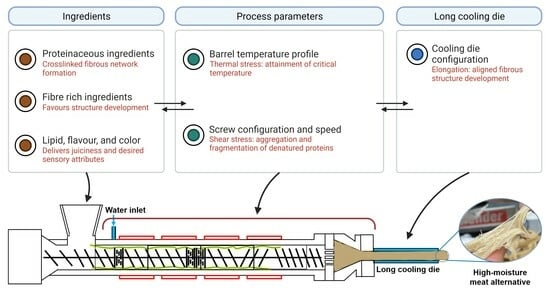
3. Effect of Process Parameters on Protein Restructuring
3.1. Temperature Profile of the Barrel
3.2. Mechanical Effects through Screw Configuration and Rotational Speed
3.3. Cooling Die Configuration
3.3.1. Slit Die
3.3.2. Annular Gap Die
3.3.3. Rotating Annular Gap Die
4. Effect of Different Ingredients on Fibrous Structure Formation
4.1. Proteinaceous Ingredients
4.1.1. Soy
4.1.2. Gluten
4.1.3. Pea
4.1.4. Faba
4.2. Other Ingredients and Their Combinations
4.3. Fibre-Rich Ingredients
4.4. Lipid, Colour, Flavour and Other Additives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glick-Bauer, M.; Yeh, M.-C. The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection. Nutrients 2014, 6, 4822–4838. [Google Scholar] [CrossRef] [PubMed]
- IFIC. Consumption Trends, Preferred Names and Perceptions of Plant-Based Meat Alternatives. 2021. Available online: https://foodinsight.org/consumption-trends-plant-based-meat-alts/ (accessed on 19 June 2022).
- Richter, C.H.; Custer, B.; Steele, J.A.; Wilcox, B.A.; Xu, J. Intensified food production and correlated risks to human health in the Greater Mekong Subregion: A systematic review. Environ. Health 2015, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Tîrziu, E.; Lazăr, R.; Sala, C.; Nichita, I.; Morar, A.; Şereş, M.; Imre, K. Salmonella in raw chicken meat from the Romanian seaside: Frequency of isolation and antibiotic resistance. J. Food Prot. 2015, 78, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef]
- Schaafsma, G. The protein digestibility–corrected amino acid score. J. Nutr. 2000, 130, 1865S–1867S. [Google Scholar] [CrossRef]
- Grossmann, L.; Weiss, J. Alternative protein sources as technofunctional food ingredients. Annu. Rev. Food Sci. Technol. 2021, 12, 93–117. [Google Scholar] [CrossRef]
- Webb, L.; Fleming, A.; Ma, L.; Lu, X. Uses of cellular agriculture in plant-based meat analogues for improved palatability. ACS Food Sci. Technol. 2021, 1, 1740–1747. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Kaplan, D.L.; Wang, Q. Protein-amylose/amylopectin molecular interactions during high-moisture extruded texturization toward plant-based meat substitutes applications. Food Hydrocoll. 2022, 127, 107559. [Google Scholar] [CrossRef]
- Immonen, M.; Chandrakusuma, A.; Sibakov, J.; Poikelispää, M.; Sontag-Strohm, T. Texturization of a Blend of Pea and Destarched Oat Protein Using High-Moisture Extrusion. Foods 2021, 10, 1517. [Google Scholar] [CrossRef]
- Jia, W.; Curubeto, N.; Rodríguez-Alonso, E.; Keppler, J.K.; van der Goot, A.J. Rapeseed protein concentrate as a potential ingredient for meat analogues. Innov. Food Sci. Emerg. Technol. 2021, 72, 102758. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, K.-X.; Guo, X.-N.; Zhou, H.-M. The impact of phosphates on the fibrous structure formation of textured wheat gluten. Food Hydrocoll. 2021, 119, 106844. [Google Scholar] [CrossRef]
- Carmo, C.S.D.; Knutsen, S.H.; Malizia, G.; Dessev, T.; Geny, A.; Zobel, H.; Myhrer, K.S.; Varela, P.; Sahlstrøm, S. Meat analogues from a faba bean concentrate can be generated by high moisture extrusion. Futur. Foods 2021, 3, 100014. [Google Scholar] [CrossRef]
- Wang, H.; Berg, F.W.v.D.; Zhang, W.; Czaja, T.P.; Zhang, L.; Jespersen, B.M.; Lametsch, R. Differences in physicochemical properties of high-moisture extrudates prepared from soy and pea protein isolates. Food Hydrocoll. 2022, 128, 107540. [Google Scholar] [CrossRef]
- Priya, R.K.; Rawson, A.; Vidhyalakshmi, R.; Mohan, R.J. Development of vegan sausage using banana floret (Musa paradisiaca) and jackfruit (Artocarpus heterophyllus Lam.) as a meat substitute: Evaluation of textural, physico-chemical and sensory characteristics. J. Food Process. Preserv. 2021, 46, e16118. [Google Scholar] [CrossRef]
- Mazlan, M.M.; Talib, R.A.; Chin, N.L.; Shukri, R.; Taip, F.S.; Nor, M.Z.M.; Abdullah, N. Physical and microstructure properties of oyster mushroom-soy protein meat analog via single-screw extrusion. Foods 2020, 9, 1023. [Google Scholar] [CrossRef]
- Ayandipe, D.O.; Adebowale, A.A.; Obadina, O.; Sanwo, K.; Kosoko, S.B.; Omohimi, C.I. Optimization of high-quality cassava and coconut composite flour combination as filler in chicken sausages. J. Culin. Sci. Technol. 2020, 20, 1–32. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Nanayakkara, R.; Pavel, L.; Robinett, T. Sensory Attributes of Jackfruit beyond Meat Sandwich Filling. J. Lifesci 2021, 2, 17–24. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, W.; Zhang, X.; Zhao, Y.; Zhang, Y.; Jiang, L.; Sui, X. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci. Technol. 2021, 109, 702–710. [Google Scholar] [CrossRef]
- Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods 2021, 10, 102. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Chen, J.; Ettelaie, R. Construction of 3D printed reduced-fat meat analogue by emulsion gels. Part II: Printing performance, thermal, tribological, and dynamic sensory characterization of printed objects. Food Hydrocoll. 2021, 121, 107054. [Google Scholar] [CrossRef]
- Chantanuson, R.; Nagamine, S.; Kobayashi, T.; Nakagawa, K. Preparation of soy protein-based food gels and control of fibrous structure and rheological property by freezing. Food Struct. 2022, 32, 100258. [Google Scholar] [CrossRef]
- Nieuwland, M.; Geerdink, P.; Brier, P.; Eijnden, P.v.D.; Henket, J.T.; Langelaan, M.L.; Stroeks, N.; van Deventer, H.C.; Martin, A.H. Reprint of “food-grade electrospinning of proteins”. Innov. Food Sci. Emerg. Technol. 2014, 24, 138–144. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Maung, T.T.; Gu, B.Y.; Kim, M.H.; Ryu, G.H. Fermentation of texturized vegetable proteins extruded at different moisture contents: Effect on physicochemical, structural, and microbial properties. Food Sci. Biotechnol. 2020, 29, 897–907. [Google Scholar] [CrossRef]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-based meat analogue (PBMA) as a sustainable food: A concise review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Ramachandraiah, K. Potential development of sustainable 3D-printed meat analogues: A review. Sustainability 2021, 13, 938. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kovis, T.J.K.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Akdogan, H. High moisture food extrusion. Int. J. Food Sci. Technol. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wei, L.; Cao, C.; Yan, W.; Wang, Q. Converting peanut protein biomass waste into “double green” meat substitutes using a high-moisture extrusion process: A multiscale method to explore a process for forming a meat-like fibrous structure. J. Agric. Food Chem. 2019, 67, 10713–10725. [Google Scholar] [CrossRef]
- Van der Sman, R.; van der Goot, A. Hypotheses concerning structuring of extruded meat analogs. Curr. Res. Food Sci. 2023, 6, 100510. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wang, Z.; Fu, L.; He, Z.; Zeng, M.; Qin, F.; Chen, J. High-moisture extrusion of soy protein: Effects of insoluble dietary fiber on anisotropic extrudates. Food Hydrocoll. 2023, 141, 108688. [Google Scholar] [CrossRef]
- Jansens, K.J.; Lagrain, B.; Rombouts, I.; Brijs, K.; Smet, M.; Delcour, J.A. Effect of temperature, time and wheat gluten moisture content on wheat gluten network formation during thermomolding. J. Cereal Sci. 2011, 54, 434–441. [Google Scholar] [CrossRef]
- Chompoorat, P.; Ambardekar, A.; Mulvaney, S.; Rayas-Duarte, P. Rheological characteristics of gluten after modified by DATEM, ascorbic acid, urea and DTT using creep-recovery test. J. Mod. Phys. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Wilde, P.J. Improving Emulsion Stability Through Selection of Emulsifiers and Stabilizers; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Czaja, T.P.; Bakalis, S.; Zhang, W.; Lametsch, R. Structural characteristics of high-moisture extrudates with oil-in-water emulsions. Food Res. Int. 2022, 158, 111554. [Google Scholar] [CrossRef] [PubMed]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Jaspe, J.; Hagen, S.J. Do Protein Molecules Unfold in a Simple Shear Flow? Biophys. J. 2006, 91, 3415–3424. [Google Scholar] [CrossRef]
- Sharma, L.G.; Pandey, L.M. Thermomechanical process induces unfolding and fibrillation of bovine serum albumin. Food Hydrocoll. 2021, 112, 106294. [Google Scholar] [CrossRef]
- Wu, M.; Huang, X.; Gao, F.; Sun, Y.; Duan, H.; Li, D. Dynamic mechanical properties and fractal analysis of texturized soybean protein/wheat gluten composite produced by high moisture extrusion. Int. J. Food Sci. Technol. 2019, 54, 499–508. [Google Scholar] [CrossRef]
- Snel, S.J.; Bellwald, Y.; van der Goot, A.J.; Beyrer, M. Novel rotating die coupled to a twin-screw extruder as a new route to produce meat analogues with soy, pea and gluten. Innov. Food Sci. Emerg. Technol. 2022, 81, 103152. [Google Scholar] [CrossRef]
- Purwanti, N.; van der Goot, A.J.; Boom, R.; Vereijken, J. New directions towards structure formation and stability of protein-rich foods from globular proteins. Trends Food Sci. Technol. 2010, 21, 85–94. [Google Scholar] [CrossRef]
- Murphy, R.P.; Riedel, Z.W.; Nakatani, M.A.; Salipante, P.F.; Weston, J.S.; Hudson, S.D.; Weigandt, K.M. Capillary RheoSANS: Measuring the rheology and nanostructure of complex fluids at high shear rates. Soft Matter 2020, 16, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- Cheftel, J.C.; Kitagawa, M.; Quéguiner, C. New protein texturization processes by extrusion cooking at high moisture levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Osen, R. Texturization of Pea Protein Isolates Using High Moisture Extrusion Cooking; University Library of the TU Munich Location: Munich, Germany, 2017; Available online: https://d-nb.info/116838026X/34 (accessed on 10 September 2023).
- Tanaka, H. Formation of Network and Cellular Structures by Viscoelastic Phase Separation. Adv. Mater. 2009, 21, 1872–1880. [Google Scholar] [CrossRef]
- Van der Sman, R.; Chakraborty, P.; Hua, N.; Kollmann, N. Scaling relations in rheology of proteins present in meat analogs. Food Hydrocoll. 2023, 135, 108195. [Google Scholar] [CrossRef]
- Cornet, S.H.; Edwards, D.; van der Goot, A.J.; van der Sman, R.G. Water release kinetics from soy protein gels and meat analogues as studied with confined compression. Innov. Food Sci. Emerg. Technol. 2020, 66, 102528. [Google Scholar] [CrossRef]
- Peng, J.; Zhu, K.-X.; Guo, X.-N.; Zhou, H.-M. Egg white protein addition induces protein aggregation and fibrous structure formation of textured wheat gluten. Food Chem. 2022, 371, 131102. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Shah, F.; Xu, Y.; Wang, Q. High-moisture extrusion of peanut protein-/carrageenan/sodium alginate/wheat starch mixtures: Effect of different exogenous polysaccharides on the process forming a fibrous structure. Food Hydrocoll. 2020, 99, 105311. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of high-moisture meat analogues with hemp and soy protein using extrusion cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Wittek, P.; Karbstein, H.P.; Emin, M.A. Blending Proteins in High Moisture Extrusion to Design Meat Analogues: Rheological Properties, Morphology Development and Product Properties. Foods 2021, 10, 1509. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-moisture meat analogues produced from yellow pea and faba bean protein isolates/concentrate: Effect of raw material composition and extrusion parameters on texture properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Martin, C. Twin screw extruders as continuous mixers for thermal processing: A technical and historical perspective. AAPS PharmSciTech 2016, 17, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Wittek, P.; Ellwanger, F.; Karbstein, H.P.; Emin, M.A. Morphology Development and Flow Characteristics during High Moisture Extrusion of a Plant-Based Meat Analogue. Foods 2021, 10, 1753. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Xia, S.; Xue, Y.; Xue, C.; Jiang, X.; Li, J. Structural and rheological properties of meat analogues from Haematococcus pluvialis residue-pea protein by high moisture extrusion. LWT 2022, 154, 112756. [Google Scholar] [CrossRef]
- Maung, T.-T.; Gu, B.-Y.; Ryu, G.-H. Influence of extrusion process parameters on specific mechanical energy and physical properties of high-moisture meat analog. Int. J. Food Eng. 2020, 17, 149–157. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein–protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Dreisoerner, J.; Wei, Y. The effects of screw configuration on the screw fill degree and special mechanical energy in twin-screw extruder for high-moisture texturised defatted soybean meal. J. Food Eng. 2015, 157, 77–83. [Google Scholar] [CrossRef]
- Wu, M.; Sun, Y.; Bi, C.; Ji, F.; Li, B.; Xing, J. Effects of extrusion conditions on the physicochemical properties of soy protein/gluten composite. Int. J. Agric. Biol. Eng. 2018, 11, 230–237. [Google Scholar] [CrossRef]
- Diaz, J.R.; Kantanen, K.; Edelmann, J.; Suhonen, H.; Sontag-Strohm, T.; Jouppila, K.; Piironen, V. Fibrous meat analogues containing oat fiber concentrate and pea protein isolate: Mechanical and physicochemical characterization. Innov. Food Sci. Emerg. Technol. 2022, 77, 102954. [Google Scholar] [CrossRef]
- Howsam, S. Multi-Channel Cooling Die. WO 01/49474 A1. 16 January 2003. Available online: https://patents.google.com/patent/CA2395185C/en?q=(cooling+die)&q=(cooling)&q=(die)&q=(plate)&q=(cross)&before=priority:20000107&scholar (accessed on 1 July 2023).
- Klein, F.; Mauchle, M.; Strässle, S. Cooling Tool for An Extruder. U.S. Patent 11,123,911, 21 September 2021. Available online: https://patents.google.com/patent/US11123911B2/en (accessed on 1 July 2023).
- Cornet, S.H.V.; Snel, S.J.E.; Schreuders, F.K.G.; van der Sman, R.G.M.; Beyrer, M.; van der Goot, A.J. Thermo-mechanical processing of plant proteins using shear cell and high-moisture extrusion cooking. Crit. Rev. Food Sci. Nutr. 2021, 62, 3264–3280. [Google Scholar] [CrossRef] [PubMed]
- Guyony, V.; Fayolle, F.; Jury, V. Die dimensions impact on fibrous plant protein formation during high moisture extrusion. Appl. Food Res. 2022, 2, 100228. [Google Scholar] [CrossRef]
- Forte, D. The Design of Food Extrusion Dies, 1st ed.; Food Industry Engineering: New York, NY, USA, 2016. [Google Scholar]
- Sengar, A.S.; Thirunavookarasu, N.; Choudhary, P.; Naik, M.; Surekha, A.; Sunil, C.; Rawson, A. Application of power ultrasound for plant protein extraction, modification and allergen reduction—A review. Appl. Food Res. 2022, 2, 100219. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Wolf, W.J. Soybean proteins. Their functional, chemical, and physical properties. J. Agric. Food Chem. 1970, 18, 969–976. [Google Scholar] [CrossRef]
- Wolf, W.; Babcock, G.; Smith, A. Purification and stability studies of the 11 S component of soybean proteins. Arch. Biochem. Biophys. 1962, 99, 265–274. [Google Scholar] [CrossRef]
- Hayashi, N.; Hayakawa, I.; Fujio, Y. Flow behaviour of soy protein isolate melt with low and intermediate moisture levels at an elevated temperature. J. Food Eng. 1993, 18, 1–11. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the specific mechanical energy on the physicochemical properties of texturized soy protein during high-moisture extrusion cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Ortolan, F.; Steel, C.J. Protein Characteristics that Affect the Quality of Vital Wheat Gluten to be Used in Baking: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Schiedt, B.; Baumann, A.; Conde-Petit, B.; Vilgis, T.A. Short- and Long-Range Interactions Governing the Viscoelastic Properties during Wheat Dough and Model Dough Development. J. Texture Stud. 2013, 44, 317–332. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Schöffel, F.; Rädle, M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of wheat gluten: Modeling of the polymerization behavior in the screw section of the extrusion process. J. Food Eng. 2018, 246, 67–74. [Google Scholar] [CrossRef]
- Domenek, S.; Morel, M.-H.; Redl, A.; Guilbert, S. Rheological investigation of swollen gluten polymer networks: Effects of process parameters on cross-link density. Macromol. Symp. 2003, 200, 137–146. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Werner, R.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of wheat gluten: Relationship between process parameters, protein polymerization, and final product characteristics. J. Food Eng. 2019, 259, 3–11. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Li, Y. Pea protein composition, functionality, modification, and food applications: A review. Adv. Food Nutr. Res. 2022, 101, 71–127. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2019, 60, 2593–2605. [Google Scholar] [CrossRef]
- Meng, Y.; Cloutier, S. Gelatin and other proteins for microencapsulation. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 227–239. [Google Scholar] [CrossRef]
- Munialo, C.D.; van der Linden, E.; Ako, K.; de Jongh, H.H. Quantitative analysis of the network structure that underlines the transitioning in mechanical responses of pea protein gels. Food Hydrocoll. 2015, 49, 104–117. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and Its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Shand, P.; Ya, H.; Pietrasik, Z.; Wanasundara, P. Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem. 2007, 102, 1119–1130. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, X.; Cheng, Y.; Cui, B.; El-Aty, A.A. Impact of high moisture contents on the structure and functional properties of pea protein isolate during extrusion. Food Hydrocoll. 2022, 127, 107508. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT Food Sci. Technol. 2021, 138, 110796. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U.; et al. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Sivasankari, R.; Hemalatha, G.; Amutha, S.; Murugan, M.; Vanniarajan, C.; Uma Maheswari, T. Extraction of protein concentrates from faba beans. Int. J. Chem. Stud. 2019, 7, 3430–3434. [Google Scholar]
- Kantanen, K.; Oksanen, A.; Edelmann, M.; Suhonen, H.; Sontag-Strohm, T.; Piironen, V.; Diaz, J.M.R.; Jouppila, K. Physical Properties of Extrudates with Fibrous Structures Made of Faba Bean Protein Ingredients Using High Moisture Extrusion. Foods 2022, 11, 1280. [Google Scholar] [CrossRef]
- Lie-Piang, A.; Braconi, N.; Boom, R.M.; van der Padt, A. Less refined ingredients have lower environmental impact—A life cycle assessment of protein-rich ingredients from oil- and starch-bearing crops. J. Clean. Prod. 2021, 292, 126046. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Shih, C.K. Rheological properties of incompatible blends of two elastomers. Polym. Eng. Sci. 1976, 16, 742–746. [Google Scholar] [CrossRef]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Kim, H.-W.; Choi, Y.-S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Korean J. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Fu, J.; Chang, Y.; Li, S.; Fang, Y. Structure Design for Improving the Characteristic Attributes of Extruded Plant-Based Meat Analogues. Food Biophys. 2021, 17, 137–149. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Zhang, Y.; Sun, C.; Fang, Y. Effect of Different Polysaccharides on the Texture and Fibrous Structure of High-Moisture Extruded Pea Protein Isolate. Food Biophys. 2023. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Zhang, B.; Drago, S.R.; Zhang, J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Synergistic effects of laccase and pectin on the color changes and functional properties of meat analogs containing beet red pigment. Sci. Rep. 2022, 12, 1168. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT 2018, 87, 546–552. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.; Ryu, G.-H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S. Removal of off-flavour-causing precursors in soy protein by concurrent treatment with phospholipase A2 and cyclodextrins. Food Chem. 2018, 264, 319–325. [Google Scholar] [CrossRef]
- Li, X.; Li, J. The Flavor of Plant-Based Meat Analogues. Cereal Foods World 2020, 65, 0040. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Yu, X.; Peng, J.; Pan, L.; Tu, K. Evaluation of the adsorption capacity and mechanism of soy protein isolate for volatile flavor compounds: Role of different oxygen-containing functional groups. Food Chem. 2022, 386, 132745. [Google Scholar] [CrossRef]
- Sambu, S.; Hemaram, U.; Murugan, R.; Alsofi, A.A. Toxicological and teratogenic effect of various food additives: An updated review. BioMed Res. Int. 2022, 2022, 6829409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: Health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2016, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Franca, P.A.P.; Duque-Estrada, P.; Sá, B.F.d.F.e.; van der Goot, A.J.; Pierucci, A.P.T.R. Meat substitutes—Past, present, and future of products available in Brazil: Changes in the nutritional profile. Future Foods 2022, 5, 100133. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Rocha, J.P.; Laster, J.; Parag, B.; Shah, N.U. Multiple Health Benefits and Minimal Risks Associated with Vegetarian Diets. Curr. Nutr. Rep. 2019, 8, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; You, J.; Zhang, H.; Xiong, S.; Yin, T.; Huang, Q. Capacity of myofibrillar protein to adsorb characteristic fishy-odor compounds: Effects of concentration, temperature, ionic strength, pH and yeast glucan addition. Food Chem. 2021, 363, 130304. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.-J.; Cheng, J.-H.; Sun, D.-W. Effects of constant power microwave on the adsorption behaviour of myofibril protein to aldehyde flavour compounds. Food Chem. 2021, 336, 127728. [Google Scholar] [CrossRef]
- Resurreccion, A. Sensory aspects of consumer choices for meat and meat products. Meat Sci. 2004, 66, 11–20. [Google Scholar] [CrossRef]


| Ingredients | Extrusion Process Parameters | Texturization/Anisotropic Index | Mixing/Kneading Elements | Effect of Extrusion Parameters | Reference |
|---|---|---|---|---|---|
| Peanut protein biomass waste | Feed rate (6 kg/h), feed moisture content (55%); Screw speed (210 rpm); Extruder barrel (red) and cooling die (blue) temperatures:  | 1.10 | 8 |
| [31] |
| EWPP and WG/S | Feed rate (10 kg/h); Screw speed (270 rpm), diameter (36 mm) and L/D (24:1); Extruder barrel (red) temperatures:  | 2.50 | - |
| [50] |
| PPI and amylose/amylopectin | Feed rate (6 kg/h); Screw speed (240 rpm) L/D (24:1); Extruder barrel (red) and cooling die (blue) temperatures:  Cooling die dimensions (70 × 10 × 1800 mm3). | - | - |
| [10] |
| SPI and PPI | Feed rate (0.3 kg/h); screw speed (200 rpm); Extruder barrel (red) and cooling die (blue) temperatures:  Cooling die dimension (5 × 20 × 200 mm3). | - | - |
| [15] |
| Pea protein powder, carrageenan, sodium alginates, and wheat starch | Feed rate (6 kg/h); feed moisture (55%); Screw speed (210 rpm); Extruder barrel (red) and cooling die (blue) temperatures:  | 1.24 | - |
| [51] |
| Hemp protein concentrate and SPI | Feed rate-solid (0.8–1.1 kg/h) and liquid (1.88–2.36 kg/h); Screw speed (300, 400, 500, 600, 800 rpm), diameter (20 mm); Extruder barrel (red) temperatures:  Cooling die dimension (25 × 7 × 300 mm3). | - | 5 |
| [52] |
| PPI and oat protein concentrate | Feed rate solid (300 g/h) and water (330 mL/h); Screw speed (300 rpm), diameter (11 cm); Extruder barrel (red) and cooling die (blue) temperatures:  Cooling die dimension (4 × 20 × 250 mm3). Cooling die dimension (4 × 20 × 250 mm3). | - |
| [11] | |
| SPI | Feed rate-solids (5 kg/h) and water (5 kg/h); Screw speed (250 rpm), L/D (29:1); Extruder barrel (red) and cooling die (blue) temperatures: For material temperature 124 °C  For material temperature 135 °C  Cooling die dimension (380 × 30 × 6 mm3). | - | 3 |
| [21] |
| SPI and whey protein concentrates | Feed rate protein blend (0.9 kg/h) and water (1.1 kg/h); Screw speed (600 rpm), diameter (11 mm), and L/D (40:1); Extruder barrel (red) and cooling die (blue) temperatures:  (Adjusted to attain a final material temperature of 115 °C, 125 °C, and 133 °C) Cooling die dimension (125 × 19 × 4 mm3). | 1.60 | 0 |
| [53] |
| Yellow PPI (both commercial and local *) and faba bean protein isolate (both commercial and local *) | Mass flow rate (2 kg/h); Screw speed (400, 600, and 800 rpm), diameter (20 mm), and L/D (40:1); Extruder barrel (red) and cooling die (blue) temperatures: Yellow PPIs  Faba bean protein isolate  Cooling die dimension (7 × 25 × 300 mm3). | - | 5 |
| [54] |
| Faba bean protein concentrate | Feed rate-product (0.95 to 1.30 kg/h) and the ratio of water-to-product feed rate (3.0 to 5.0); Screw speed (900 rpm); Extruder barrel (red) and cooling die (blue) temperatures:  Cooling die dimension (30 × 5 × 300 mm3). Cooling die dimension (30 × 5 × 300 mm3). | 4 × Forward mixing element; 1 × Reverse mixing element |
| [14] |
| Protein Ingredient | Structuring Ingredients, Flavouring and Other Ingredients | Extrusion Moisture Content/Feed Rate | Product Description and Image (of Best Resultant Combination) | Reference |
|---|---|---|---|---|
| WG (9 portions), EWPP (0.25, 0.5, 0.75, 1, and 2%) | Wheat starch (1 portion) | 30% |
 | [50] |
| WG (9 portions) | Starch (1 portion), phosphates (sodium tripolyphosphate, sodium pyrophosphate, sodium hexametaphosphate) (0, 0.25, 0.5, 1, and 2%) | 30% |
 | [13] |
| PPI (9 portions) | Amylose (1 portion)/amylopectin (1 portion) | 58% |
 | [10] |
| SPI/PPI (19.5%) and WG (19.5%) | Sodium chloride (1%) | 60% |
 | [77] |
| SPI (100, 95, 90, 80, 70, 60, and 50%) | Microalgae powder (0, 5, 10, 20, 30, 40, and 50%) | 55, 60, and 65% |
 | [58] |
| Soy protein concentrate (planetary roller extruder) | Iota (ι) carrageenan (ICGN) (0, 0.75, 1.5, 2.25, and 3%) | 10 kg/h |
 | [103] |
| Isolated soy protein (50%), WG (40%) | Corn starch (10%) | 700 g/kg |
 | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengar, A.S.; Beyrer, M.; McDonagh, C.; Tiwari, U.; Pathania, S. Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives. Foods 2023, 12, 3830. https://doi.org/10.3390/foods12203830
Sengar AS, Beyrer M, McDonagh C, Tiwari U, Pathania S. Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives. Foods. 2023; 12(20):3830. https://doi.org/10.3390/foods12203830
Chicago/Turabian StyleSengar, Animesh Singh, Michael Beyrer, Ciara McDonagh, Uma Tiwari, and Shivani Pathania. 2023. "Effect of Process Variables and Ingredients on Controlled Protein Network Creation in High-Moisture Plant-Based Meat Alternatives" Foods 12, no. 20: 3830. https://doi.org/10.3390/foods12203830