1. Introduction
Beef, which is rich in amino acids and various trace elements, is highly sought after by consumers [
1]. Currently, the massive consumption of beef is second only to that of pork and poultry, and as the quality of life of consumers is improving, the demand for high-quality beef is increasing [
2]. According to data, the worldwide demand for beef was 70 million tons in 2019; it is estimated that the global demand for beef in 2023 will be approximately 74 million tons, with an the increase of approximately 4 million tons over the four years. The beef cattle industry is playing a crucial role in the global livestock farming industry [
3]. However, the development of the beef cattle industry still needs to be improved regarding industry efficiency, meat quality, and ecological sustainability [
4,
5]. Improving the nutrition of livestock feed through an appropriate mix of feeds during the production and breeding process can be a cost-effective way to improve the production performance of beef cattle and meat quality [
6].
Roughage is a crucial component of ruminant feeds, and its high crude fiber content is degraded and fermented in the rumen by rumen microbiota and then utilized by the body [
7,
8]. Peanut vine is a typical by-product of peanuts, and it can be used as a quality roughage resource [
9]. It is estimated that the yield of peanut vine after peanut harvest accounts for about 60% to 65% of the total peanut production [
10]. The crude protein (CP) content of peanut vine is about 12%, it is rich in vitamins and minerals [
11], and its neutral detergent fiber (NDF) and acid detergent fiber (ADF) are about 50% and 36%, and are more easily digestible than alfalfa [
12]. Quality CP in the diet is easy to digest and degrade, which promotes the synthesis of rumen microbial protein and the absorption of nutrients by the body [
13]. Zhu et al. found that supplementing peanut vine in roughage for beef cattle compared to wheat straw could increase the CP content and linolenic acid level of longissimus dorsi muscle [
14]. Abdou et al. found that the supplementation of peanut vine in millet stover diets for ruminants improved production performance and effectively reduced feeding costs [
15]. Corn silage is an important source of fiber and energy for ruminants [
16], with the protein content of about 8.8%, NDF content of about 50.3%, and ADF content of about 26.0% [
17]. Zaralis et al. found that using corn silage as the sole forage in bull diets enhanced live weight gain [
18]. A study has shown that supplementing corn silage to cattle diets compared to grass silage can increase dry matter (DM) intake and slightly increase carcass gain [
19]. Phipps et al. found that supplementing corn silage in cow diets compared to first-cut perennial ryegrass silage increased forage DM intake and milk yield [
20]. Therefore, supplementing ruminant diets with peanut vine or corn silage can increase the productivity of ruminants. It has been found that interactive effects exist in feeds for animals. A good combination of feeds would create more positive effects, which in turn would improve the efficiency of feed utilization [
21].
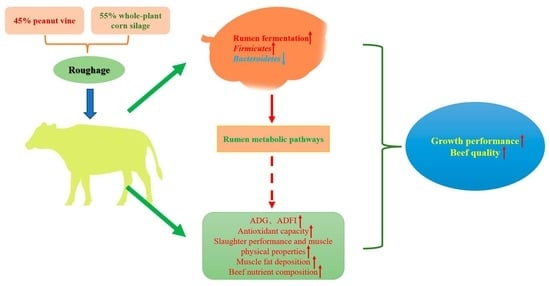
We assumed that the diet combining 45% peanut vine and 55% whole-plant corn silage in roughage would enhance growth performance and meat quality of Simmental crossbred cattle. Accordingly, this research investigates the effects of the diet combining peanut vine and whole-plant corn silage on the growth performance, meat quality, and rumen microbiota of Simmental crossbred cattle.
4. Discussion
The study aimed to investigate the effects of diets combining peanut vine and whole-plant corn silage on beef cattle. Roughage is an extremely important component of ruminant feeds, and high-quality roughage can improve the efficiency of ruminant farming, as well as the composition of it making a significant influence on the growth performance of cattle [
29]. We found that diets combining peanut vine and whole-plant corn silage improved the growth performance of beef cattle, especially the ADG of the MPG group which was the highest; this result was similar to Abdou et al. [
15]. This is probably due to the lower nutritional value of the roughage in the WG group, such as the much lower CP content compared to the LPG, MPG and HPG groups [
30], making it difficult to provide sufficient nutrients for ruminants. Moreover, the ratio of peanut vine to whole-plant corn silage in the MPG group can have a greater positive combination effect and improve the production performance of the cattle [
21]. On the other hand, the growth performance of the MPG group was higher than that of the WG group, which may be due to the higher fiber and lignin content of wheat straw compared to peanut vine, which can decrease the degradation of it by rumen microbiota, thus reducing feed digestion and utilization by the body. However, the organ indexes of the treatment groups indicated that the supplementation of peanut vine in the roughage of cattle would not affect the growth and development of organs.
Antioxidation is an active and spontaneous self-protection mechanism of the animal body, and antioxidant enzymes can enhance the immunity and defense of the body and reduce the damage caused by free radicals [
31]. The activities of T-AOC, GSH-Px, SOD, and MDA can be used to reflect the antioxidant capacity of the animal. T-AOC is a comprehensive indicator of antioxidant capacity [
32]. GSH-Px and SOD are two important antioxidant enzymes whose main role is eliminating free radicals in the body [
33]. MDA can damage the integrity of tissues and cells, which can lead to cell damage. In this study, the SOD activity in spleen of the MPG group was higher than that of the WG group. This result shows the diet combining 45% peanut vine and 55% whole-plant corn silage improved the antioxidant capacity of the body. It is probably due to some bioactive components such as flavonoids in peanut vine that improve the antioxidant capacity [
34], and it could also be one of the reasons that the diet combining peanut vine and whole-plant corn silage improves the performance of the cattle.
Slaughter performance is an essential indicator in the beef industry. Our study found that the MPG group was higher than the other groups with regard to carcass weight, net meat weight, and eye muscle area, indicating that the diet combining 45% peanut vine and 55% whole-plant corn silage could improve the slaughter performance of cattle. It could be due to the increased ADFI and ADG of the beef cattle in the MPG group, which thus increased the digestion of the feed by the body, and improved nutrient deposition in the body. On the other hand, it could be due to the MPG group’s roughage combination producing more positive effects.
Changes in EE and CP content in muscle can affect the sensory properties and nutritional traits of beef. In this study, we found that the DM, CP and EE content in the muscle of the MPG group was significantly higher than that of the WG group. The results indicate that the diet combining 45% peanut vine and 55% whole-plant corn silage produced a more positive effect, enhanced the absorption of nutrients from the feed by the body, and thus promoted the deposition of nutrients in the muscle. Interestingly, we found that the LPG group had the highest EE content in muscle of the treatment groups and that the supplementation of 25% peanut vine in the roughage might be the optimum ratio to promote muscle fat deposition in cattle. Linoleic acid, linolenic acid, and arachidonic acid as PUFA in the muscle have been found to reduce cardiovascular diseases, lower cholesterol, and reduce hypertension [
35]. We found that the content of linoleic acid in the muscle of the LPG and HPG groups was higher than that of the WG group. This is probably because the PUFA content of wheat straw, which belongs to graminoid forage, is lower than that contained in peanut vine, resulting in differences in the linoleic acid content of beef among the treatment groups [
14]. As an essential organ of the body, the liver is where most of the metabolic activities of the body, such as the oxidation of fatty acids and the synthesis of lipids such as triglycerides and cholesterol, take place. FAS, MDH, and LPL are crucial enzymes involved in lipid metabolism in the body. FAS is a crucial enzyme in the de novo synthesis of fatty acids, and its activity can be used to determine the capacity of fatty acid synthesis in the liver. MDH is involved in the tricarboxylic acid cycle in the body and plays essential roles in the synthesis of fatty acids. In contrast, LPL is a crucial enzyme in lipolysis metabolism, which is mainly secreted and expressed by adipocytes, myocytes, and macrophages [
36]. We found that cattle in the LPG group increased the gene expression levels of
FAS and
MDH2. The results indicate that the supplementation of appropriate amounts of peanut vine can promote the synthesis of fatty acids, thus increasing the fat deposition in the liver, which can further increase triglycerides and cholesterol levels in the liver to promote the deposition of fat in the muscle. Interestingly, the beef cattle in the LPG group also increased the gene expression level of
LPL, which in turn accelerated fat catabolism. From our results, it seems that the diet combining 25% peanut vine and 75% whole-plant corn silage would eventually promote fat deposition, probably due to the efficiency of lipid synthesis being higher than that of lipolysis in the body.
Ruminal pH reflects the state of rumen fermentation, which is around 6.6 in normal conditions. Mould et al. found that rumen degradation of cellulose was decreased when the pH in the rumen was below 6.0, thereby reducing the digestibility of the feed [
37]. We found the rumen pH was in the normal range in all groups, indicating that feeding different ratios of peanut vine to whole-plant corn silage combinations cannot affect ruminal pH in the cattle. NH
3-N is a degradation product of protein, amino acids, and non-protein nitrogen in the feed, reflecting the use of ammonia by rumen microbiota and the efficiency of the nitrogen degradation in the rumen. However, excessively high NH
3-N concentration would cause nitrogen losses, and excessively low NH
3-N concentration would reduce rumen degradation of fiber and the productivity of rumen microbial protein synthesis. In this study, NH
3-N concentration ranged from 6.95 to 12.08 mg/dl, within the normal range for all groups. The NH
3-N concentration in the MPG group was significantly lower than that in the WG group. However, the rate of protein degradation in the rumen was affected by the quality of CP in the diet. The result might be due to the fact that the quality of CP in the diet combining peanut vine and whole-plant corn silage was greater than that in the diet combining wheat straw and whole-plant corn silage, which accelerated the degradation of protein in the rumen, and facilitated the synthesis of bacterial protein by the rumen microbiota using NH
3-N, thus providing energy for the growth of the body, which laterally explains the higher ADG in the MPG group. Ruminal VFA is produced by the fermentation of carbohydrates in the feed by rumen microbiota [
38]. VFA can provide the body with energy and can also participate in various physiological processes such as the tricarboxylic acid cycle, the transformation and storage of glucose, and the formation of lipid, thus affecting overall health and performance. Raspa et al. found that a high-fiber diet significantly influenced VFA content in the horse gut, which is different from the results of the present study. This finding indicates that differences in animal species, fermentation sites, and feed sources can influence the VFA content in the body [
39]. The A/P ratio can reflect the utilization of nutrients by the body of the animal. We found that the A/P ratio of all treatment groups was within the normal range in this study, and the A/P ratios of the LPG and MPG groups were lower than those of the HPG group, which were more beneficial to nutrient absorption and utilization. It is easy to see that the diet combining 45% peanut vine and 55% whole-plant corn silage improved rumen fermentation.
The rumen microbiota can degrade the majority of organic and fibrotic material in feed and plays essential roles in the nutritional metabolism, digestion, and absorption of ruminants. We found no significant differences in microbial α-diversity in all the treatments of diets combining peanut vine and whole-plant corn silage. At the phylum level, the dominant phylum of rumen microbiota were
Firmicutes,
Bacteroidetes, and
Tenericute in all the treatments, and the relative abundances of
Firmicutes and
Bacteroidetes were higher. Chang et al. found that
Firmicutes and
Bacteroidetes were higher in relative abundance in the rumen of cattle [
40], which is similar to this study. Furthermore, Myer et al. found
Firmicutes and
Bacteroidetes as the two dominant bacterial phyla in the rumen of ruminants [
41]. It was found that the relative levels of
Firmicutes and
Bacteroidetes could be used to assess the energy requirements of ruminants [
42].
Firmicutes can produce a diverse range of lipases, proteases, cellulases, etc., thereby hydrolyzing complex macromolecular compounds such as fats, cellulose, and glycans [
43].
Bacteroidetes are generally regarded as having a role in degrading such proteins and carbohydrates [
44]. It was reported that the relative abundance of
Firmicutes was higher than that of
Bacteroidetes in obese people and obese mice. The obese mice fecal bacteria transplantation to the germ-free mice increased in fat deposition, so
Firmicutes may be related to lipid metabolism in the body [
45]. We found the abundance of
Firmicutes gradually increased, and the abundance of
Bacteroidetes gradually decreased at the phylum level with increasing supplementation of peanut vine in the roughage. This suggests that diets combining peanut vine and whole-plant corn silage increased the abundance of
Firmicutes, promoted the deposition of body fat, and improved the production performance of beef cattle.
In-depth analysis of the rumen microbiota found that the dominant genera were mainly
Prevotella_1 and
Ruminococcus_2 at the genus level in all the treatment groups. During the fermentation process in the rumen,
Prevotella_1 is one of the most commonly abundant genera. It acts as the main protein-degrading bacteria in the rumen to digest proteins, peptides, and starch-active substances. It cannot degrade fiber directly but can coexist with fiber-degrading bacteria to indirectly promote fiber degradation and plays essential roles in the metabolism in the rumen [
46]. We found that the relative abundance of Prevotella_1 in the rumen of the treatments was lower than that of the WG group, but it was still the dominant bacteria in the rumen, similar to the results of Jami et al. [
47]. This may be due to the higher content of degradable fiber in the diets combining peanut vine and whole-plant corn silage, which affects the rumen microbiota and increases the abundance of fiber-degrading bacteria, thereby affecting the relative abundance of the
Prevotella [
48].
Ruminococcus is a group of fiber-degrading bacteria in the rumen which plays a major role in fiber degradation and can degrade cellulose and hemicellulose in plant-based feed [
49]. We found that the relative abundance of
Ruminococcus_2 in the rumen of the HPG group was significantly higher than that of the WG group, which is likely to be related to the increase in degradable fiber content in the feed. Pitta et al. found that
Fibrobacter and
Ruminococcus had a tendency to increase in the rumen of cattle with increased amounts of hay [
50]. The results were similar to this study. The relative abundance of
Ruminococcus_2 was lower in the WG group. This may be due to the higher content of difficult to degrade fiber such as lignin in the diet combining wheat straw and whole-plant corn silage, which affects the colonization of fiber-degrading bacteria in the rumen, thus causing changes in the rumen microbiota. But the higher abundance of
Prevotella_1 in the rumen can be used to assist the degradation of fiber so that the body obtains sufficient nutrients. In addition, Ley et al. found that
Prevotella was associated with inflammation in the body [
51]. In this study, the growth performance of the WG group was poorer than that of the peanut vine treatment groups, probably due to the higher relative abundance of
Prevotella_1 in the WG group, which affected the health status of the body, which in turn affected the digestion and utilization of various nutrients by the rumen. The rumen is a crucial digestive organ for ruminants, with more than 70% of the feed consumed being degraded by the microbiota in the rumen and eventually digested and absorbed by the body. Saleem et al. argued that about 55–60% of rumen metabolites are related to rumen microbes [
52], and the microbes are closely linked to muscle fat deposition in the body. Correlation analysis of rumen microbes with rumen metabolites and lipid metabolism genes showed that
Prevotella_1 was positively correlated with acetate level, which is probably because
Prevotella can effectively degrade xylan, xyloglucan, and pectin and translates sugars into acetate, succinate, and propionate [
53]. The main metabolic function of microbes that participate in the metabolic processes of the host by utilizing carbohydrates hardly utilized and absorbed by the host itself, plays a crucial role in rumen metabolism. Therefore, PICRUSt analysis was carried out based on the results of rumen microbial sequencing and found that functions such as lipid metabolism and membrane transport were up-regulated in the treatments that were fed the diets combining peanut vine and whole-plant corn silage. It is probably because
Ruminococcus and other fiber-degrading bacteria can degrade fiber and produce acetate, propionate, and butyrate in the rumen. VFA can improve carbohydrate utilization, promoting lipid metabolism in the body. In correlation analysis of the predicted functional pathways and bacteria, we found that
Ruminococcus_2 was positively correlated with carbohydrate metabolism, while
Ruminococcus belongs to the group of fiber-degrading bacteria that can hydrolyze and ferment carbohydrates which plays essential roles in rumen fermentation, further confirming our findings. Moreover, we found that
Prevotella_1 was positively correlated with glycan biosynthesis and metabolism, demonstrating that
Prevotella plays essential roles in the digestion and utilization of glycans and plant polysaccharides.