Core-Shell-Shell Upconversion Nanomaterials Applying for Simultaneous Immunofluorescent Detection of Fenpropathrin and Procymidone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
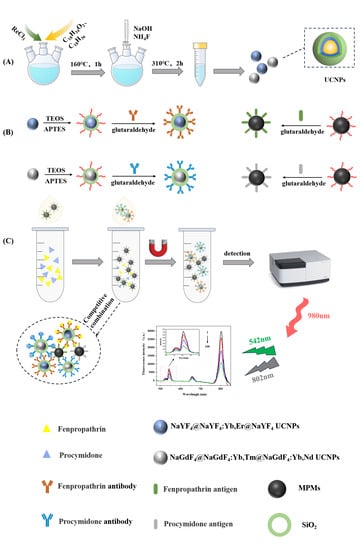
2.2. Synthesis of NaGdF4@NaGdF4: Yb, Tm@NaGdF4: Yb, Nd UCNPs
2.3. Modification of Upconversion Nanomaterials
2.4. Preparation and Optimization of Capture Probe and Signal Probe
2.5. Optimization of the Addition Amount of Capture Probes
2.6. Establishment of Upconversion Magnetic Separation Immunofluorescence Detection Method
2.7. Method Specificity
2.8. Actual Sample Testing
3. Results and Discussion
3.1. Characterization of Upconversion Nanomaterials
3.2. Optimization of Antigen and Antibody Addition Amount
3.3. Optimization of the Amount of Capture Probe Addition
3.4. Establishment of Upconversion Magnetic Separation Immunofluorescence Detection Method
3.5. Method Specificity
3.6. Matrix Impact Elimination and Addition Recovery Experiment
3.7. Actual Sample Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Y.K.; Li, S.; Hong, M.; Wang, Z.G.; Ji, G.N.; Chen, R.P.; Zhao, X.D.; Wang, Y.; Ren, S.Y.; Han, D.P.; et al. Synthesis and functionalization of upconversion nano materials and their application in food safety detection. Anal. Chem. 2021, 49, 1955–1969. [Google Scholar]
- Hakeem, D.A.; Su, S.S.; Mo, Z.R.; Wen, H.L. Upconversion luminescent nanomaterials: A promising new platform for food safety analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 8866–8907. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Jeon, E.; Kim, S.; Lee, J.S. Lanthanide-doped upconversion nanomaterials: Recent advances and applications. Biochip. J. 2020, 14, 124–135. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.L.; Yao, X.T.; Xu, X.K.; Wei, H.P.; Liu, Y.L.; Zhuang, J.L. Recent progress of rare earth upconversion luminescent materials/quantum dots composite materials. J. Chin. Soc. Rare Earths 2021, 39, 827–839. [Google Scholar]
- Fan, Y.; Liu, L.; Zhang, F. Exploiting lanthanide-doped upconversion nanoparticles with core/shell structures. Nano Today 2019, 25, 68–84. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhao, Y.H.; Hu, R.; Lin, G.M.; Qu, J.L.; Liu, L.W. Upconversion nanoparticles: Mechanisms, synthesis and biology applications. J. Chin. Soc. Rare Earths 2022, 40, 533–549. [Google Scholar]
- Borse, S.; Rafique, R.; Murthy, Z.V.P.; Park, T.J.; Kailasa, S.K. Applications of upconversion nanoparticles in analytical and biomedical sciences: A review. Analyst 2022, 147, 3155–3179. [Google Scholar] [CrossRef]
- Ji, G.N.; Wang, Y.; Qin, Y.K.; Peng, Y.; Li, S.; Han, D.P.; Ren, S.Y.; Qin, K.; Li, S.; Gao, Z.X.; et al. Latest developments in the upconversion nanotechnology for the rapid detection of food safety: A review. Nanotechnol. Rev. 2022, 11, 2101–2122. [Google Scholar] [CrossRef]
- Rong, Y.; Hassan, M.M.; Ouyang, Q.; Chen, Q. Lanthanide ion (Ln3+)-based upconversion sensor for quantification of food contaminants: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3531–3578. [Google Scholar] [CrossRef]
- Qin, Y.K.; Li, S.; Wang, Y.; Ren, S.Y.; Han, D.P.; Peng, Y.; Zhou, H.Y.; Gao, Z.X.; Han, T. Simultaneous determination of ochratoxin A and zearalenone in maize and oat flour by magnetically controlled Bi-color upconversion fluorescent aptasensor. J. Food Saf. Food Qual. 2022, 13, 2580–2588. [Google Scholar]
- Su, D.; Zhao, X.; Yan, X.; Han, X.; Zhu, Z.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; Liu, X. Background-free sensing platform for on-site detection of carbamate pesticide through upconversion nanoparticles-based hydrogel suit. Biosens. Bioelectron. 2021, 194, 113598–113605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Xu, D.M.; Hou, J.B.; Zhang, Y.Q.; Zhu, Z.L.; Mao, R.Y.; Qiu, H.; Xie, W.; Shen, W.J.; Yi, X.H. Research method of rapid determination of chiral pesticide fenpropathrin enantiomers in fruit and vegetable puree by supercritical fluid chromatography. J. Sep. Sci. 2022, 45, 2717–2723. [Google Scholar] [CrossRef]
- Mi, Y.; Jia, C.; Lin, X.; Liu, X.; Lu, R. Dispersive solid-phase extraction based on β-cyclodextrin grafted hyperbranched polymers for determination of pyrethroids in environmental water samples. Microchem. J. 2019, 150, 104164. [Google Scholar] [CrossRef]
- Di, S.S.; Wang, Y.H.; Xu, H.; Wang, X.Q.; Yang, G.L.; Chen, C.; Yang, X.; Qian, Y.Z. Comparison the dissipation behaviors and exposure risk of carbendazim and procymidone in greenhouse strawberries under different application method: Individual and joint applications. Food Chem. 2021, 354, 129502. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Tomigahara, Y.; Tarui, H.; Omori, R.; Kawamura, S. Identification of metabolism and excretion differences of procymidone between Rats and humans using chimeric mice: Implications for differential developmental toxicity. J. Agric. Food Chem. 2018, 66, 1955–1963. [Google Scholar] [CrossRef]
- Tekin, Z.; Zaman, B.T.; Bakirdere, S. Rapid, accurate and sensitive determination of fenpropathrin as insecticide in dried strawberry samples by high performance liquid chromatography, and in vivo stability and behavior under gastric conditions. Chem. Lett. 2020, 49, 17–19. [Google Scholar] [CrossRef]
- Li, G.Z.; Sun, J.S.; Li, J.H.; Zhang, Y.L.; Huang, J.C.; Yue, F.L.; Dong, H.W.; Li, F.L.; Xu, H.H.; Guo, Y.Y.; et al. Paper-based biosensors relying on core biological immune scaffolds for the detection of procymidone in vegetables. Talanta 2023, 265, 124843. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.T.; Dou, C.X.; Li, H.H. The present situation of pesticide residues and their laboratory detection technology in vegetables & fruits. China Food Saf. Mag. 2021, 3, 115–117. [Google Scholar]
- Bi, S.Y.; Li, B.L.; Zhu, Z.Q.; Cao, T.; Gan, H.; Jin, H. Determination of 7 commonly used pesticide residues in citrus fruits by QuEChERS-GC-ECD. Food Ind. 2019, 40, 305–308. [Google Scholar]
- Luo, Y. Determination of pythiril, fenvalerate, fenpropathrin and pentachloronitrobenzene residues in soil by solid phase extraction GC-ECD method. Chem. Eng. 2020, 34, 28–30. [Google Scholar]
- Zhao, L.Y.; Jin, J.Y.; Zhu, W.B.; Zuo, Y.H.; Song, Y. Detection of pyrethroids in food by immunofluorescence enhanced method based on three-layer core-shell structure upconversion materials. Foods 2022, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- GB/T 20769-2008; Determination of 450 Pesticides and Related Chemicals Residues in Fruits and Vegetables—LC-MS-MS Method. Available online: https://max.book118.com/html/2019/0107/7052156036002000.shtm (accessed on 26 August 2023).
- Deng, L.H. Development of Immunoassay for Tadalafil and Its Analogues in Health-Care Food. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- GB 2763-2021; National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. Available online: http://down.foodmate.net/wap/index.php?itemid=97819&moduleid=23 (accessed on 26 August 2023).







| Sample | Pesticide | Additive Concentration (μg/kg) | Fluorescence Immunoassay | HPLC | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD (μg/kg) | Recovery Rate (%) | CV (%) | Mean ± SD (μg/kg) | Recovery Rate (%) | CV (%) | |||
| Tomato | Fenpropathrin | 2.5 | 2.16 ± 0.12 | 86.50 | 5.5 | 2.22 ± 0.18 | 88.93 | 7.9 |
| 10 | 9.23 ± 0.37 | 92.27 | 4.0 | 9.23 ± 0.32 | 92.25 | 3.4 | ||
| 25 | 25.05 ± 0.47 | 100.20 | 1.9 | 24.91 ± 0.34 | 99.63 | 1.4 | ||
| Procymidone | 2.5 | 2.30 ± 0.13 | 91.89 | 5.8 | 2.35 ± 0.14 | 93.87 | 6.1 | |
| 10 | 9.20 ± 0.24 | 92.02 | 2.6 | 8.99 ± 0.35 | 89.88 | 3.9 | ||
| 25 | 24.76 ± 0.58 | 99.06 | 2.3 | 24.78 ± 0.30 | 99.11 | 1.2 | ||
| Cucumber | Fenpropathrin | 2.5 | 2.40 ± 0.12 | 96.03 | 5.2 | 2.40 ± 0.14 | 96.13 | 5.8 |
| 10 | 8.97 ± 0.44 | 89.68 | 4.9 | 9.47 ± 0.41 | 94.67 | 4.3 | ||
| 25 | 23.28 ± 0.68 | 93.11 | 2.9 | 23.12 ± 0.40 | 92.49 | 1.7 | ||
| Procymidone | 2.5 | 2.18 ± 0.17 | 87.27 | 7.7 | 2.20 ± 0.16 | 87.87 | 7.2 | |
| 10 | 9.96 ± 0.41 | 99.57 | 4.2 | 9.45 ± 0.31 | 94.53 | 3.3 | ||
| 25 | 25.61 ± 0.68 | 102.43 | 2.7 | 25.68 ± 0.61 | 102.73 | 2.4 | ||
| Cabbage | Fenpropathrin | 5 | 4.28 ± 0.42 | 89.30 | 8.8 | 4.11 ± 0.22 | 82.13 | 5.3 |
| 20 | 18.24 ± 1.15 | 96.44 | 3.4 | 18.85 ± 0.92 | 94.27 | 4.9 | ||
| 50 | 48.49 ± 0.87 | 94.03 | 2.1 | 46.49 ± 0.97 | 92.97 | 2.1 | ||
| Procymidone | 5 | 4.28 ± 0.42 | 85.61 | 9.7 | 4.41 ± 0.30 | 88.13 | 6.9 | |
| 20 | 18.24 ± 1.15 | 91.24 | 6.3 | 17.71 ± 0.83 | 88.53 | 4.7 | ||
| 50 | 48.49 ± 0.87 | 96.97 | 1.8 | 46.83 ± 0.98 | 93.67 | 2.1 | ||
| Sample | Fluorescence Immunoassay | HPLC | ||
|---|---|---|---|---|
| Fenpropathrin | Procymidone | Fenpropathrin | Procymidone | |
| (μg/L) | (μg/L) | (μg/L) | (μg/L) | |
| 1–4# Tomato | - | - | - | - |
| 5# Tomato | 8.7 | 8.2 | ||
| 6–10# Cucumber | - | - | - | - |
| 11# Cabbage | - | 12.8 | - | 13.5 |
| 12# Cabbage | - | - | - | - |
| 13# Cabbage | - | 42.1 | 41.5 | |
| 14–15# Cabbage | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Jin, J.; Hu, L.; Hu, B.; Wang, M.; Guo, L.; Lv, X. Core-Shell-Shell Upconversion Nanomaterials Applying for Simultaneous Immunofluorescent Detection of Fenpropathrin and Procymidone. Foods 2023, 12, 3445. https://doi.org/10.3390/foods12183445
Song Y, Jin J, Hu L, Hu B, Wang M, Guo L, Lv X. Core-Shell-Shell Upconversion Nanomaterials Applying for Simultaneous Immunofluorescent Detection of Fenpropathrin and Procymidone. Foods. 2023; 12(18):3445. https://doi.org/10.3390/foods12183445
Chicago/Turabian StyleSong, Yang, Jingyi Jin, Liuling Hu, Bingqian Hu, Mengyao Wang, Lilong Guo, and Xiyan Lv. 2023. "Core-Shell-Shell Upconversion Nanomaterials Applying for Simultaneous Immunofluorescent Detection of Fenpropathrin and Procymidone" Foods 12, no. 18: 3445. https://doi.org/10.3390/foods12183445