Sucrose Esters and Beeswax Synergize to Improve the Stability and Viscoelasticity of Water-in-Oil Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Oil Dispersions and W/O Emulsions
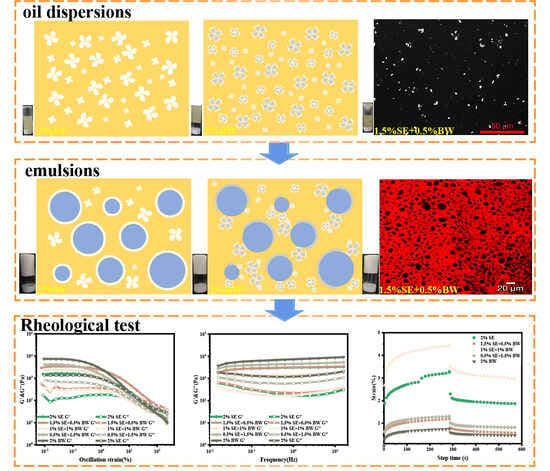
2.3. Microstructure Observation
2.4. X-ray Diffraction (XRD) Analysis
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Rheological Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Related Characterization of Oil Dispersions
3.2. Related Characterization of Emulsions
3.2.1. Appearance and Microstructure of W/O Emulsions
3.2.2. Rheological Properties of W/O Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Colucci, G.; Santamaria-Echart, A.; Silva, S.C.; Fernandes, I.P.M.; Sipoli, C.C.; Barreiro, M.F. Development of Water-in-Oil Emulsions as Delivery Vehicles and Testing with a Natural Antimicrobial Extract. Molecules 2020, 25, 2105. [Google Scholar] [CrossRef]
- Negrini, N.C.; Lipreri, M.V.; Tanzi, M.C.; Farè, S. In vitro cell delivery by gelatin microspheres prepared in water-in-oil emulsion. J. Mater. Sci. Mater. Med. 2020, 31, 26. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Z.; Lu, X. Modulation of starch nanoparticle surface characteristics for the facile construction of recyclable Pickering interfacial enzymatic catalysis. Green Chem. 2019, 21, 2412–2427. [Google Scholar] [CrossRef]
- Iqbal, S.; Hameed, G.; Baloch, M.K.; McClements, D.J. Structuring lipids by aggregation of acidic protein microspheres in W/O emulsions. LWT-Food Sci. Technol. 2013, 51, 16–22. [Google Scholar] [CrossRef]
- Smulek, W.; Jarzebski, M. Hemp seed oil nanoemulsion with Sapindus saponins as a potential carrier for iron supplement and vitamin D. Rev. Adv. Mater. Sci. 2023, 62, 20220317. [Google Scholar] [CrossRef]
- Bharti, D.; Banerjee, I.; Cerqueira, M.A.; Kim, D.; Pal, K. The potential role of hydrophilic and hydrophobic liquid emulsifier-tailored sunflower wax/sunflower oil oleogels on the properties of whole wheat batter and sponge cakes. Int. J. Food Eng. 2023, 19, 301–313. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Y.; Jia, X.; Li, J.; Zhang, M.; Yin, L. Review on the Stability Mechanism and Application of Water-in-Oil Emulsions Encapsulating Various Additives. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1660–1675. [Google Scholar] [CrossRef]
- Bastida-Rodríguez, J. The Food Additive Polyglycerol Polyricinoleate (E-476): Structure. Appl. Prod. Methods 2013, 2013, 124767. [Google Scholar]
- Golodnizky, D.; Davidovich-Pinhas, M. The Effect of the HLB Value of Sucrose Ester on Physiochemical Properties of Bigel Systems. Foods 2020, 9, 1857. [Google Scholar] [CrossRef]
- Szuts, A.; Szabo-Revesz, P. Sucrose esters as natural surfactants in drug delivery systems-A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef]
- Rincon-Cardona, J.A.; Agudelo-Laverde, L.M.; Martini, S.; Candal, R.J.; Herrera, M.L. In situ synchrotron radiation X-ray scattering study on the effect of a stearic sucrose ester on polymorphic behavior of a new sunflower oil variety. Food Res. Int. 2014, 64, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; McClements, D.J. Lemon oil solubilization in mixed surfactant solutions: Rationalizing microemulsion & nanoemulsion formation. Food Hydrocoll. 2012, 26, 268–276. [Google Scholar]
- Hu, X.; Binks, B.P.; Cui, Z. Water-in-oil Pickering emulsions stabilized by edible surfactant crystals formed in situ. Food Hydrocoll. 2022, 125, 107394. [Google Scholar] [CrossRef]
- Martini, S.; Carelli, A.A.; Lee, J. Effect of the Addition of Waxes on the Crystallization Behavior of Anhydrous Milk Fat. J. Am. Oil Chem. Soc. 2008, 85, 1097–1104. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Mauricio-Perez, R.; Gonzalez-Chavez, M.M.; Sanchez-Becerril, M.; de Jesus Ornelas-Paz, J.; Perez-Martinez, J.D. Physical properties of organogels and water in oil emulsions structured by mixtures of candelilla wax and monoglycerides. Food Res. Int. 2013, 54, 1360–1368. [Google Scholar] [CrossRef]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Effect of Water Addition on Physical Properties of Emulsion Gels. Food Biophys. 2019, 14, 30–40. [Google Scholar] [CrossRef]
- Ogutcu, M.; Arifoglu, N.; Yilmaz, E. Preparation and Characterization of Virgin Olive Oil-Beeswax Oleogel Emulsion Products. J. Am. Oil Chem. Soc. 2015, 92, 459–471. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, Q.; Chen, J.; Ye, T.; Fan, L.; Li, J. Fabrication and characterization of oleogels and temperature-responsive water-in-oil emulsions based on candelilla (Euphorbia cerifera) wax. Food Chem. 2022, 397, 133677. [Google Scholar] [CrossRef]
- Meng, Z.; Guo, Y.; Wang, Y.; Liu, Y. Oleogels from sodium stearoyl lactylate-based lamellar crystals: Structural characterization and bread application. Food Chem. 2019, 292, 134–142. [Google Scholar] [CrossRef]
- Jana, S.; Martini, S. Effect of High-Intensity Ultrasound and Cooling Rate on the Crystallization Behavior of Beeswax in Edible Oils. J. Agric. Food Chem. 2014, 62, 10192–10202. [Google Scholar] [CrossRef]
- Sanchez-Becerril, M.; Marangoni, A.G.; Perea-Flores, M.J.; Cayetano-Castro, N.; Martinez-Gutierrez, H.; Andraca-Adame, J.A.; Perez-Martinez, J.D. Characterization of the micro and nanostructure of the candelilla wax organogels crystal networks. Food Struct. -Neth. 2018, 16, 1–7. [Google Scholar] [CrossRef]
- Lupi, F.R.; De Santo, M.P.; Ciuchi, F.; Baldino, N.; Gabriele, D. The role of edible oils in low molecular weight organogels rheology and structure. Food Res. Int. 2018, 111, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Babaahmadi, M.; Lesaffer, A.; Dewettinck, K. Rheological Profiling of Organogels Prepared at Critical Gelling Concentrations of Natural Waxes in a Triacylglycerol Solvent. J. Agric. Food Chem. 2015, 63, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Lei, Y.; Wu, Y.; Liang, H.; Li, J.; Pei, Y.; Li, Y.; Li, B.; Luo, X.; Liu, S. Beeswax: A potential self-emulsifying agent for the construction of thermal-sensitive food W/O emulsion. Food Chem. 2021, 349, 129203. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Zheng, Z.; Chai, X.; Han, W.; Liu, Y. Gelation behavior and crystal network of natural waxes and corresponding binary blends in high-oleic sunflower oil. J. Food Sci. 2021, 86, 3987–4000. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Cao, L.-L.; Pang, M.; Hou, Z.-G.; Jiang, S.-T. Preparation of camellia oil-based W/O emulsions stabilized by tea polyphenol palmitate: Structuring camellia oil as a potential solid fat replacer. Food Chem. 2019, 276, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Wijarnprecha, K.; de Vries, A.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Microstructure and rheology of oleogel-stabilized water-in-oil emulsions containing crystal-stabilized droplets as active fillers. LWT-Food Sci. Technol. 2019, 115, 108058. [Google Scholar] [CrossRef]
- Freitas, G.B.; Duncke, A.C.; Barbato, C.N.; de Oliveira, M.C.K.; Pinto, J.C.; Nele, M. Influence of wax chemical structure on W/O emulsion rheology and stability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 45–56. [Google Scholar] [CrossRef]
- Lu, Y.; Qian, X.; Xie, W.; Zhang, W.; Huang, J.; Wu, D. Rheology of the sesame oil-in-water emulsions stabilized by cellulose nanofibers. Food Hydrocoll. 2019, 94, 114–127. [Google Scholar] [CrossRef]
- Manoi, K.; Rizvi, S.S.H. Emulsification mechanisms and characterizations of cold, gel-like emulsions produced from texturized whey protein concentrate. Food Hydrocoll. 2009, 23, 1837–1847. [Google Scholar] [CrossRef]
- Botega, D.C.Z.; Nogueira, C.; de Moura, N.M.; Martinez, R.M.; Rodrigues, C.; Barrera-Arellano, D. Influence of Aqueous Matrices into Candelilla Wax Organogels Emulsions for Topical Applications. J. Am. Oil Chem. Soc. 2021, 98, 317–328. [Google Scholar] [CrossRef]
- Li, X.; Fan, L.; Li, J. Extrusion-based 3D printing of high internal phase emulsions stabilized by co-assembled beta-cyclodextrin and chitosan. Food Hydrocoll. 2023, 134, 108036. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, R.; Song, Z.; Zhang, S.; Zhao, X.; Jiang, J.; Liu, Y. Freeze-thaw stability and oil crystallization behavior of phospholipids/whey protein-costabilized acidic emulsions with four oil types. Food Hydrocoll. 2022, 125, 107385. [Google Scholar] [CrossRef]
- Anvari, M.; Joyner, H.S. Effect of fish gelatin and gum arabic interactions on concentrated emulsion large amplitude oscillatory shear behavior and tribological properties. Food Hydrocoll. 2018, 79, 518–525. [Google Scholar] [CrossRef]
- Gu, X.; Du, L.; Meng, Z. Comparative study of natural wax-based W/O emulsion gels: Microstructure and macroscopic properties. Food Res. Int. 2023, 165, 112509. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, X.-Y.; Li, D.; Wang, L.-J.; Wang, Y. Development of soy protein isolate emulsion gels as extrusion-based 3D food printing inks: Effect of polysaccharides incorporation. Food Hydrocoll. 2022, 131, 107824. [Google Scholar] [CrossRef]
- Du, L.; Meng, Z. Oleofoams and emulsion foams stabilized by sodium stearoyl lactylate: Insight into their relations based on microstructure, rheology and tribology. Food Hydrocoll. 2023, 137, 108317. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, M.; Zhou, Y.; Liu, Y.; Fan, L.; Li, J. Sucrose Esters and Beeswax Synergize to Improve the Stability and Viscoelasticity of Water-in-Oil Emulsions. Foods 2023, 12, 3387. https://doi.org/10.3390/foods12183387
Shu M, Zhou Y, Liu Y, Fan L, Li J. Sucrose Esters and Beeswax Synergize to Improve the Stability and Viscoelasticity of Water-in-Oil Emulsions. Foods. 2023; 12(18):3387. https://doi.org/10.3390/foods12183387
Chicago/Turabian StyleShu, Mingjun, Yuling Zhou, Yuanfa Liu, Liuping Fan, and Jinwei Li. 2023. "Sucrose Esters and Beeswax Synergize to Improve the Stability and Viscoelasticity of Water-in-Oil Emulsions" Foods 12, no. 18: 3387. https://doi.org/10.3390/foods12183387