Supercritical CO2 Processing of White Grape Must as a Strategy to Reduce the Addition of SO2
Abstract
:1. Introduction
2. Materials and Methods
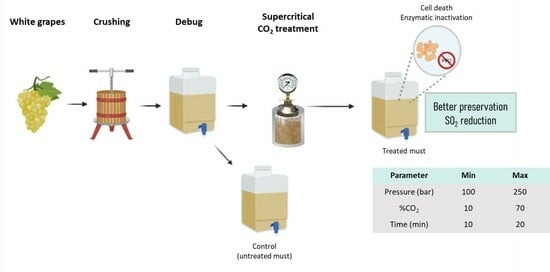
2.1. Must Preparation
2.2. Supercritical Fluid Treatment
2.3. Must Characterization
2.3.1. Oenological Parameters of the Musts
2.3.2. Total Polyphenol Index (TPI)
2.3.3. Antioxidant Capacity
2.3.4. Polyphenol Oxidase Activity (PPO)
2.3.5. Microbiological Analysis
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameters
3.1.1. Effects of the HPCD on the Treated Must
- The CO2 solubilization in the extracellular medium causes a decrease in the pH that contributes to the permeabilization of the membrane and the inhibition of microorganism growth.
- The cell membrane modification, due to an easy penetration of CO2 through the lipidic membrane. At this point, CO2 can combine with the phospholipid layer creating cavities that cause a structural and functional disorder, increasing its permeability.
- Penetration of the CO2 into the cytoplasm. The H+ protons generated are buffered in order to maintain the intracellular pH, but if the accumulation is too high, cells could be unable to maintain the pH homeostasis and the pH decreases.
- Enzyme modification and metabolic interference for the pH decrease, causing cell death and decreasing enzyme solubility. Moreover, the reduction of the enzyme activity is linked to the denaturation phenomena due to the modification of their secondary and tertiary structure [38].
- Metabolic interference of carbonic acid, involved in carboxylation and decarboxylation reactions.
- Disorder of the electrolyte balance inside the cell. The carbonic acid inside the cell is converted to carbonate and causes Ca+2 and Mg+2 precipitation from cell membranes.
- Extraction of constituents from the cell membrane and cytoplasm. CO2 increases the intracellular density and causes the dissolution of constituents, especially phospholipids and hydrophobic compounds, and transfers them to the extracellular environment.

3.1.2. Determining the Influence of the Process Variables
3.1.3. Total Polyphenol Index and Antioxidant Capacity
3.2. Inertization Capacity of the Supercritical Treatment
3.2.1. PPO Inactivation
3.2.2. Microbial Inactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Salehi, F. Physico-chemical properties of fruit and vegetable juices as affected by pulsed electric field: A review. Int. J. Food Prop. 2020, 23, 1036–1050. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology—The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Morgan, S.C.; Tantikachornkiat, M.; Scholl, C.M.; Benson, N.L.; Cliff, M.A.; Durall, D.M. The effect of sulfur dioxide addition at crush on the fungal and bacterial communities and the sensory attributes of pinot gris wines. Int. J. Food Microbiol. 2019, 290, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Raposo, R.; Moreno-Rojas, J.M.; Zafrilla, P.; Cayuela, J.M.; Mulero, J.; Puertas, B.; Guerrero, R.F.; Piñeiro, Z.; Giron, F.; et al. Efficacy of olive oil mill extract in replacing sulfur dioxide in wine model. LWT-Food Sci. Technol. 2015, 61, 117–123. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.; Chen, X.; Wu, H.; Xiao, X.; Xie, L.; Wei, Z.; Xiong, R.; Zhou, X. Effects of plant-derived polyphenols on the antioxidant activity and aroma of sulfur-dioxide-free red wine. Molecules 2023, 28, 5255. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, Y.; Sun, Z.; Li, X. Chitooligosaccharide as a possible replacement for sulfur dioxide in winemaking. Appl. Sci. 2020, 10, 578. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef]
- Divol, B.; Strehaiano, P.; Lonvaud-Funel, A. Effectiveness of dimethyldicarbonate to stop alcoholic fermentation in wine. Food Microbiol. 2005, 22, 169–178. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef]
- Bertolini, F.M.; Morbiato, G.; Facco, P.; Marszałek, K.; Pérez-Esteve, É.; Benedito, J.L.; Zambon, A.; Spilimbergo, S. Optimization of the supercritical CO2 pasteurization process for the preservation of high nutritional value of pomegranate juice. J. Supercrit. Fluids 2020, 164, 104914. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative hurdle technologies for the preservation of functional fruit juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical carbon dioxide technology: A promising technique for the non-thermal processing of freshly fruit and vegetable juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Raso, J.; Álvarez, I.; Delso, C.; Morata, A. Pulsed electric fields to improve the use of non-saccharomyces starters in red wines. Foods 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem 2020, 331, 127259. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W.; Górecki, A.; Błaszczak, W.; Lamparski, G.; Amarowicz, R.; Fornal, J.; Jeliński, T. Effects of high hydrostatic pressure processing on the physicochemical and sensorial properties of a red wine. Innov. Food Sci. Emerg. Technol. 2012, 16, 409–416. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Rocha, M.A.M.; Rodrigues, A.; Rocha, S.M.; Saraiva, J.A.; Coimbra, M.A. Impact of high pressure treatments on the physicochemical properties of a sulphur dioxide-free white wine during bottle storage: Evidence for maillard reaction acceleration. Innov. Food Sci. Emerg. Technol. 2013, 20, 51–58. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Morata, A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by high hydrostatic pressure: Effect on use of non-saccharomyces in must fermentation. Food Bioprocess Technol. 2016, 9, 1769–1778. [Google Scholar] [CrossRef]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef]
- Chang, Y.H.; Wu, S.J.; Chen, B.Y.; Huang, H.W.; Wang, C.Y. Effect of high-pressure processing and thermal pasteurization on overall quality parameters of white grape juice. J. Sci. Food Agric. 2017, 97, 3166–3172. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Bañuelos, M.A.; Puig-Pujol, A.; Guamis, B.; González, C.; Suárez-Lepe, J.A. Use of ultra-high pressure homogenization processing in winemaking: Control of microbial populations in grape musts and effects in sensory quality. Innov. Food Sci. Emerg. Technol. 2018, 50, 50–56. [Google Scholar] [CrossRef]
- van Wyk, S.; Farid, M.M.; Silva, F.V.M. SO2, high pressure processing and pulsed electric field treatments of red wine: Effect on sensory, brettanomyces inactivation and other quality parameters during one year storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 204–211. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Trigueros, E.; Beltrán, S.; Melgosa, R. Effect of high pressure carbon dioxide on tomato juice: Inactivation kinetics of pectin methylesterase and polygalacturonase and determination of other quality parameters. J. Food Eng. 2018, 239, 64–71. [Google Scholar] [CrossRef]
- Marszałek, K.; Skąpska, S.; Woźniak, Ł.; Sokołowska, B. Application of supercritical carbon dioxide for the preservation of strawberry juice: Microbial and physicochemical quality, enzymatic activity and the degradation kinetics of anthocyanins during storage. Innov. Food Sci. Emerg. Technol. 2015, 32, 101–109. [Google Scholar] [CrossRef]
- Oulé, K.M.; Dickman, M.; Arul, J. Properties of orange juice with supercritical carbon dioxide treatment. Int. J. Food Prop. 2013, 16, 1693–1710. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Mena-Morales, A.; Pérez-Navarro, J.; García-Romero, E.; Cejudo-Martín de Almagro, V.M.; Guri-Baiget, S.; Mallén-Pomes, J. Saturation of grape musts with CO2: A technique to reduce the use of SO2 in white wines. LWT 2021, 152, 112318. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a natural antioxidant from grape pomace extract in the development of bioactive jute fibers for food packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Arruda, H.S.; Eberlin, M.N.; Pastore, G.M.; Meireles, M.A.A. Effects of supercritical carbon dioxide and thermal treatment on the inulin chemical stability and functional properties of prebiotic-enriched apple juice. Food Res. Int. (Ott. Ont.) 2019, 125, 108561. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.M.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Kanavouras, A.; Coutelieris, F.; Karanika, E.; Kotseridis, Y.; Kallithraka, S. Color change of bottled white wines as a quality indicator. OENO One 2020, 54, 543–551. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Analytical Standards of the Adolfo Lutz Institute: Physicochemical Methods for Food Analysis; Instituto Adolfo Lutz: Sao Paulo, Brazil, 2008; Volume IV. [Google Scholar]
- University of La Rioja. Available online: https://www.unirioja.es/color/descargas.shtml (accessed on 10 August 2023).
- Ribereau-Gayon, J. Le dosage des composés phénoliques totaux dans les vins rouges. Chem.-Anal. 1970, 52, 4. [Google Scholar]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (aai) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Maturin, L.; Peeler, J.T. Chapter 3: Aerobic plate count. In Bacteriological Analytical Manual (Bam); FDA: Silver Spring, MD, USA, 2001. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count (accessed on 10 August 2023).
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast kluyveromyces thermotolerans isolated in greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Ribeiro, N.; Soares, G.C.; Santos-Rosales, V.; Concheiro, A.; Alvarez-Lorenzo, C.; García-González, C.A.; Oliveira, A.L. A new era for sterilization based on supercritical CO2 technology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 399–428. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, C.; Balaban, M.; Benedito, J. Modelling of the inactivation kinetics of escherichia coli, saccharomyces cerevisiae and pectin methylesterase in orange juice treated with ultrasonic-assisted supercritical carbon dioxide. J. Supercrit. Fluids 2014, 90, 18–26. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.F.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous nacl solutions from 273 to 533 k and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Zhou, L.; Bi, X.; Xu, Z.; Yang, Y.; Liao, X. Effects of high-pressure CO2 processing on flavor, texture, and color of foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Briongos, H.; Illera, A.E.; Sanz, M.T.; Melgosa, R.; Beltrán, S.; Solaesa, A.G. Effect of high pressure carbon dioxide processing on pectin methylesterase activity and other orange juice properties. LWT 2016, 74, 411–419. [Google Scholar] [CrossRef]
- Chater, J.M.; Merhaut, D.J.; Jia, Z.; Arpaia, M.L.; Mauk, P.A.; Preece, J.E. Effects of site and cultivar on consumer acceptance of pomegranate. J. Food Sci. 2018, 83, 1389–1395. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Beltrán, S.; Melgosa, R.; Solaesa, A.G.; Ruiz, M.O. Evaluation of hpcd batch treatments on enzyme inactivation kinetics and selected quality characteristics of cloudy juice from golden delicious apples. J. Food Eng. 2018, 221, 141–150. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Linhu, Z.; Liu, Y.; Xu, X.; Pan, S.; Hu, W. Effect of high-pressure carbon dioxide on the aggregation and conformational changes of polyphenol oxidase from apple (Malus domestica) juice. Innov. Food Sci. Emerg. Technol. 2019, 54, 43–50. [Google Scholar] [CrossRef]
- Niu, S.; Xu, Z.; Fang, Y.; Zhang, L.; Yang, Y.; Liao, X.; Hu, X. Comparative study on cloudy apple juice qualities from apple slices treated by high pressure carbon dioxide and mild heat. Innov. Food Sci. Emerg. Technol. 2010, 11, 91–97. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Leng, X.; Liao, X.; Hu, X. Acceleration of precipitation formation in peach juice induced by high-pressure carbon dioxide. J. Agric. Food Chem. 2010, 58, 9605–9610. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.Y.; Lee, H.J.; Kim, S.A.; Rhee, M.S. Inactivation of alicyclobacillus acidoterrestris spores in apple juice by supercritical carbon dioxide. Int. J. Food Microbiol. 2009, 136, 95–100. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Kim, H.J.; Kim, J.S.; Kim, J.S. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci. Biotechnol. 2019, 28, 1607–1615. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, Q.; Zhang, R.; Wei, Z.; Deng, Y.; Zhang, Y.; Tang, X.; Zhang, M. Comparative study on phenolic profiles and antioxidant activity of litchi juice treated by high pressure carbon dioxide and thermal processing. Food Sci. Technol. Res. 2015, 21, 41–49. [Google Scholar] [CrossRef]
- Kaushik, N.; Kaur, B.P.; Rao, P.S.; Mishra, H.N. Effect of high pressure processing on color, biochemical and microbiological characteristics of mango pulp (Mangifera indica cv. Amrapali). Innov. Food Sci. Emerg. Technol. 2014, 22, 40–50. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Hu, W.; Liao, X. Changes in the activity, dissociation, aggregation, and the secondary and tertiary structures of a thaumatin-like protein with a high polyphenol oxidase activity induced by high pressure CO2. Innov. Food Sci. Emerg. Technol. 2014, 23, 68–78. [Google Scholar] [CrossRef]
- Pozo-Insfran, D.d.; Balaban, M.O.; Talcott, S.T. Inactivation of polyphenol oxidase in muscadine grape juice by dense phase-CO2 processing. Food Res. Int. 2007, 40, 894–899. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Wang, Y.; Bi, X.; Buckow, R.; Liao, X. Effects of high pressure CO2 treatments on microflora, enzymes and some quality attributes of apple juice. J. Food Eng. 2011, 104, 577–584. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Barba, F.J.; Skąpska, S.; Lorenzo, J.M.; Zambon, A.; Spilimbergo, S. Enzymatic, physicochemical, nutritional and phytochemical profile changes of apple (Golden delicious L.) juice under supercritical carbon dioxide and long-term cold storage. Food Chem. 2018, 268, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ortuño, C.; Quiles, A.; Benedito, J. Inactivation kinetics and cell morphology of e. Coli and s. Cerevisiae treated with ultrasound-assisted supercritical CO2. Food Res. Int. 2014, 62, 955–964. [Google Scholar] [CrossRef]
- Chen, J.l.; Zhang, J.; Song, L.; Jiang, Y.; Wu, J.; Hu, X.S. Changes in microorganism, enzyme, aroma of hami melon (Cucumis melo L.) juice treated with dense phase carbon dioxide and stored at 4 °C. Innov. Food Sci. Emerg. Technol. 2010, 11, 623–629. [Google Scholar] [CrossRef]
- Santos Júnior, L.C.O.d.; Heberle, I.; Aquino, A.C.M.d.S.; Oliveira, J.V.; Ribeiro, D.H.B.; Medeiros, J.d.D.; Amante, E.R. High-pressure supercritical carbon dioxide uses to inactivate escherichia coli in pumpkin puree. Res. Soc. Dev. 2021, 10, e6510413853. [Google Scholar] [CrossRef]






| Experiment Number | P (bar) | % CO2 | Vsample (mL) | t (min) |
|---|---|---|---|---|
| 1 | 100 | 10 | 90 | 10 |
| 2 | 100 | 10 | 90 | 20 |
| 3 | 100 | 40 | 60 | 10 |
| 4 | 100 | 40 | 60 | 20 |
| 5 | 100 | 70 | 30 | 10 |
| 6 | 100 | 70 | 30 | 20 |
| 7 | 250 | 10 | 90 | 10 |
| 8 | 250 | 10 | 90 | 20 |
| 9 | 250 | 40 | 60 | 10 |
| 10 | 250 | 40 | 60 | 20 |
| 11 | 250 | 70 | 30 | 10 |
| 12 | 250 | 70 | 30 | 20 |
| Color Intensity | Acidity | |
|---|---|---|
| Untreated must—10% CO2 | nd | nd |
| Untreated must—40% CO2 | nd | nd |
| Untreated must—70% CO2 | nd | nd |
| 10% CO2–40% CO2 | nd | nd |
| 10% CO2–70% CO2 | * | * |
| 40% CO2–70% CO2 | * | * |
| Pressure | % CO2 | Time | Log UFC/mL Reduction | D-Value (min) |
|---|---|---|---|---|
| 100 | 10 | 10 | 2.6 ± 0.5 | 4.0 ± 0.8 |
| 10 | 20 | 2.9 ± 0.0 | 6.7 ± 0 | |
| 40 | 10 | 3.5 ± 0.7 | 5.9 ± 1.2 | |
| 40 | 20 | <LoD | <LoD | |
| 70 | 10 | <LoD | <LoD | |
| 70 | 20 | <LoD | <LoD | |
| 250 | 10 | 10 | <LoD | <LoD |
| 10 | 20 | <LoD | <LoD | |
| 40 | 10 | <LoD | <LoD | |
| 40 | 20 | <LoD | <LoD | |
| 70 | 10 | <LoD | <LoD | |
| 70 | 20 | <LoD | <LoD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejudo, C.; Díaz, A.B.; Casas, L.; Martínez de la Ossa, E.; Mantell, C. Supercritical CO2 Processing of White Grape Must as a Strategy to Reduce the Addition of SO2. Foods 2023, 12, 3085. https://doi.org/10.3390/foods12163085
Cejudo C, Díaz AB, Casas L, Martínez de la Ossa E, Mantell C. Supercritical CO2 Processing of White Grape Must as a Strategy to Reduce the Addition of SO2. Foods. 2023; 12(16):3085. https://doi.org/10.3390/foods12163085
Chicago/Turabian StyleCejudo, Cristina, Ana Belén Díaz, Lourdes Casas, Enrique Martínez de la Ossa, and Casimiro Mantell. 2023. "Supercritical CO2 Processing of White Grape Must as a Strategy to Reduce the Addition of SO2" Foods 12, no. 16: 3085. https://doi.org/10.3390/foods12163085