Algae Incorporation and Nutritional Improvement: The Case of a Whole-Wheat Pasta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
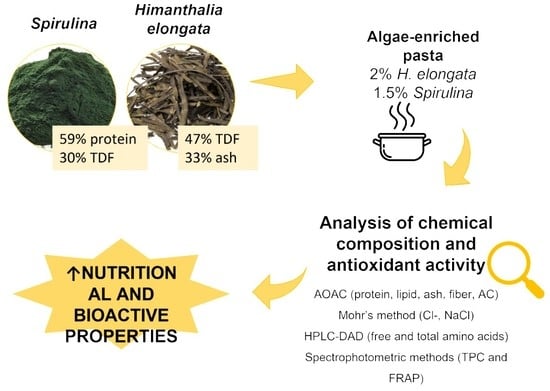
2.2. Samples and Sample Preparation
2.3. Nutritional Analysis
2.4. Amino Acid Profile Analysis
2.4.1. Free Amino Acid Extraction
2.4.2. Total Amino Acid Extraction
2.4.3. Chromatographic Separation and Detection
2.5. Total Phenolic Content and In Vitro Antioxidant Activity
2.5.1. Extract Preparation
2.5.2. Total Phenolics Content (TPC)
2.5.3. Ferric Reducing Antioxidant Power (FRAP)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of the Algae and Pasta
3.2. Total Phenolic Content and Antioxidant Activity
3.3. Amino Acid Profile
3.3.1. Total Amino Acids
3.3.2. Free Amino Acids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gohara-Beirigo, A.K.; Matsudo, M.C.; Cezare-Gomes, E.A.; Carvalho, J.C.; Danesi, E.D. Microalgae trends toward functional staple food incorporation: Sustainable alternative for human health improvement. Trends Food Sci. Technol. 2022, 125, 185–199. [Google Scholar] [CrossRef]
- Geyik, O.; Hadjikakou, M.; Bryan, B.A. Spatiotemporal trends in adequacy of dietary nutrient production and food sources. Glob. Food Secur. 2020, 24, 100355. [Google Scholar] [CrossRef]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key challenges for the commercial expansion of ingredients from algae into human food products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Eggertsen, M.; Halling, C. Knowledge gaps and management recommendations for future paths of sustainable seaweed farming in the Western Indian Ocean. Ambio 2021, 50, 60–73. [Google Scholar] [CrossRef] [Green Version]
- van Oirschot, R.; Thomas, J.B.E.; Gröndahl, F.; Fortuin, K.P.J.; Brandenburg, W.; Potting, J. Explorative environmental life cycle assessment for system design of seaweed cultivation and drying. Algal Res. 2017, 27, 43–54. [Google Scholar] [CrossRef]
- Algamar. Available online: https://algamar.com/pt-pt/quem-somos/ (accessed on 27 July 2023).
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef]
- Medeiros, V.P.B.; Costa, W.K.A.; Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pages, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullon, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef]
- Subbiah, V.; Xie, C.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A. The quest for phenolic compounds from seaweed: Nutrition, biological activities, and applications. Food Rev. Int. 2022, 1–28. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2373–2391. [Google Scholar] [CrossRef]
- Din, N.A.S.; Alayudin, S.M.; Sofian-Seng, N.S.; Rahman, H.A.; Razali, N.S.M.; Lim, S.J.; Mustapha, W.A.W. Brown algae as functional food source of fucoxanthin: A review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Ejike, C.E.C.C.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Mohamed, I.M.A.; Osman, A.I.; Rooneyet, D.W. Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: A review. Environ. Chem. Lett. 2023, 21, 97–152. [Google Scholar] [CrossRef]
- Raczyk, M.; Polanowska, K.; Kruszewski, B.; Grygier, A.; Michalowska, D. Effect of Spirulina (Arthrospira platensis) supplementation on physical and chemical properties of semolina (Triticum durum) based fresh pasta. Molecules 2022, 27, 355. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products. Part 1: Preparation and evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbie, S. Nutritional, functional, textural, and sensory evaluation of spirulina enriched green pasta: A potential dietary and health supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef]
- Hussein, A.; Ibrahim, G.; Kamil, M.; El-Shamarka, M.; Mostafa, S.; Mohamed, D. Spirulina-enriched pasta as functional food rich in protein and antioxidant. Biointerface Res. Appl. Chem. 2021, 11, 14736–14750. [Google Scholar]
- Ramírez-Rodrigues, M.M.; Estrada-Beristain, C.; Metri-Ojeda, J.; Pérez-Alva, A.; Baigts-Allende, D.K. Spirulina platensis protein as sustainable ingredient for nutritional food products development. Sustainability 2021, 13, 6849. [Google Scholar] [CrossRef]
- Fradinho, P.; Niccolai, A.; Soares, R.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Effect of Arthrospira platensis (spirulina) incorporation on the rheological and bioactive properties of gluten-free fresh pasta. Algal Res. 2020, 45, 101743. [Google Scholar] [CrossRef]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J. Food Qual. 2019, 2019, 3707219. [Google Scholar] [CrossRef] [Green Version]
- Grahl, S.; Strack, M.; Mensching, A.; Mörlein, D. Alternative protein sources in Western diets: Food product development and consumer acceptance of spirulina-filled pasta. Food Qual. Prefer. 2020, 84, 103933. [Google Scholar] [CrossRef]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Rico, D.; Linaje, A.A.; Herrero, A.; Asensio-Vegas, C.; Miranda, J.; Martínez-Villaluenga, C.; Luis, D.A.; Martin-Diana, A.B. Carob by-products and seaweeds for the development of functional bread. J. Food Process. Preserv. 2018, 42, 13700. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Enhancement of the phytochemical and fibre content of beef patties with Himanthalia elongata seaweed. Int. J. Food Sci. Technol. 2013, 48, 2239–2249. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MA, USA, 2019; Volume 1. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors. In Food and Nutrition Paper 77; FAO: Rome, Italy, 2002. [Google Scholar]
- Machado, S.; Costa, A.S.; Pimentel, F.; Oliveira, M.B.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Costa, A.S.; Torres, D.; Almeida, M.F.; Oliveira, M.B. Phenylketonuria: Protein content and amino acids profile of dishes for phenylketonuric patients. The relevance of phenylalanine. Food Chem. 2014, 149, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish powdered seaweeds: Composition, antioxidant capacity and technological properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerryet, J.P. An assessment of selected nutritional, bioactive, thermal and technological properties of brown and red Irish seaweed species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Aouir, A.; Amiali, M.; Bitam, A.; Benchabane, A.; Raghavan, V.G. Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. J. Food Meas. Charact. 2017, 11, 913–923. [Google Scholar] [CrossRef]
- Aljobair, M.O.; Albaridi, N.A.; Alkuraieef, A.N.; AlKehayez, N.M. Physicochemical properties, nutritional value, and sensory attributes of a nectar developed using date palm puree and spirulina. Int. J. Food Prop. 2021, 24, 845–858. [Google Scholar] [CrossRef]
- van der Zanden, L.D.; van Kleef, E.; de Wijk, R.A.; van Trijp, H.C. Knowledge, perceptions and preferences of elderly regarding protein-enriched functional food. Appetite 2014, 80, 16–22. [Google Scholar] [CrossRef]
- Martin, C.; Morel, M.H.; Reau, A.; Cuq, B. Kinetics of gluten protein-insolubilisation during pasta processing: Decoupling between time- and temperature-dependent effects. J. Cereal Sci. 2019, 88, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Lemes, A.C.; Takeuchi, K.P.; Carvalho, J.C.M.; Danesi, E.D.G. Fresh pasta production enriched with Spirulina platensis biomass. Braz. Arch. Biol. Technol. 2012, 55, 741–750. [Google Scholar] [CrossRef]
- Graça, C.; Fradinho, P.; Sousa, I.; Raymundo, A. Impact of Chlorella vulgaris on the rheology of wheat flour dough and bread texture. LWT-Food Sci. Technol. 2018, 89, 466–474. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for protein. EFSA J. 2012, 10, 2557. [Google Scholar] [CrossRef]
- Belda, M.; Sanchez, D.; Bover, E.; Prieto, B.; Padrón, C.; Cejalvo, D.; Lloris, J.M. Extraction of polyphenols in Himanthalia elongata and determination by high performance liquid chromatography with diode array detector prior to its potential use against oxidative stress. J. Chromatogr. B 2016, 1033–1034, 334–341. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia Elongata from Western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Gargouri, M.; Magne, C.; Feki, A. Hyperglycemia, oxidative stress, liver damage and dysfunction in alloxan-induced diabetic rat are prevented by Spirulina supplementation. Nutr. Res. 2016, 36, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Karray, A.; Krayem, N.; Saad, H.B.; Sayari, A. Spirulina platensis, Punica granatum peel, and moringa leaves extracts in cosmetic formulations: An integrated approach of in vitro biological activities and acceptability studies. Environ. Sci. Pollut. Res. 2021, 28, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.R.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Gupta, S.; Rajauria, G.; Abu-Ghannam, N. Study of the microbial diversity and antimicrobial properties of Irish edible brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.C.; Pereira, L.; Goncalves, A.M.M. Environmental impact on seaweed phenolic production and activity: An important step for compound exploitation. Mar. Drugs 2021, 19, 245. [Google Scholar] [CrossRef]
- Kapoor, S.; Singh, M.; Srivastava, A.; Chavali, M.; Chandrasekhar, K.; Verma, P. Extraction and characterization of microalgae-derived phenolics for pharmaceutical applications: A systematic review. J. Basic Microbiol. 2022, 62, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Pan-Utai, W.; Iamtham, S.; Boonbumrung, S.; Mookdasanit, J. Improvement in the sequential extraction of phycobiliproteins from Arthrospira platensis using green technologies. Life 2022, 12, 1896. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, J.O.; Käding, S.-C.; Niewalda, I.R.; Most, E.; Dorigam, J.C.P.; Eder, K. The influence of dietary leucine above recommendations and fixed ratios to isoleucine and valine on muscle protein synthesis and degradation pathways in broilers. Poult. Sci. 2019, 98, 6772–6786. [Google Scholar] [CrossRef] [PubMed]
- Gorissen, S.H.M.; Phillips, S.M. Branched-chain amino acids (leucine, isoleucine, and valine) and skeletal muscle. In Nutrition and Skeletal Muscle; Walrand, S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 283–298. [Google Scholar]
- Gutierrez-Salmean, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar] [PubMed]
- Tantawy, A.A.; Naguib, D.M. Arginine, histidine and tryptophan: A new hope for cancer immunotherapy. PharmaNutrition 2019, 8, 100149. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2016, 29, 1001–1009. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- Engelking, L. Amino Acid Catabolism. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382. [Google Scholar] [CrossRef]
| Nutritional Composition | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | % Dry Weight | % Dry Weight | % Fresh Weight | |||||||
| SPI | HIM | RP | RPA | CP | CPA | CP | CPA | |||
| Moisture | - | - | - | - | - | - | 58.6 ± 0.2 | 56.9 ± 0.3 | * | |
| Protein | 59.2 ± 0.5 | 8.8 ± 0.3 | * | 10.1 ± 0.4 c | 13.1 ± 0.4 a | 11.8 ± 0.6 b | 13.5 ± 0.4 a | 6.9 ± 0.3 | 7.7 ± 0.2 | * |
| Fat | 1.10 ± 0.03 | 0.14 ± 0.00 | * | 0.27 ± 0.01 d | 0.46 ± 0.02 b | 0.55 ± 0.02 a | 0.37 ± 0.00 c | 0.32 ± 0.01 | 0.21 ± 0.00 | * |
| TDF | 29.7 ± 0.4 | 47.2 ± 1.8 | * | 9.2 ± 0.1 a | 10.3 ± 0.5 a | 10.2 ± 0.7 a | 10.5 ± 0.5 a | 6.0 ± 0.4 | 6.0 ± 0.3 | |
| IDF | 12.8 ± 0.6 | 18.0 ± 1.0 | * | 6.7 ± 0.2 a | 5.5 ± 0.1 b | 4.8 ± 0.3 b | 5.3 ± 0.3 b | 2.8 ± 0.2 | 3.0 ± 0.2 | |
| SDF | 16.9 ± 0.9 | 29.2 ± 0.8 | * | 2.5 ± 0.1 b | 4.9 ± 0.5 a | 5.0 ± 1.0 a | 5.2 ± 0.2 a | 3.2 ± 0.6 | 3.0 ± 0.1 | |
| AC | 2.8 ± 0.6 | 11.0 ± 1.1 | * | 78.7 ± 0.2 a | 74.0 ± 1.0 b | 75.2 ± 0.5 ab | 73.3 ± 0.4 b | 44.2 ± 0.3 | 41.8 ± 0.3 | * |
| Ash | 7.1 ± 0.0 | 32.5 ± 0.6 | * | 1.70 ± 0.10 c | 2.15 ± 0.02 b | 2.19 ± 0.02 ab | 2.37 ± 0.04 a | 1.28 ± 0.01 | 1.35 ± 0.02 | * |
| Cl− | 0.25 ± 0.04 | 9.9 ± 0.1 | * | 0.26 ± 0.04 a | 0.23 ± 0.00 a | 0.23 ± 0.00 a | 0.26 ± 0.04 a | 0.14 ± 0.00 | 0.15 ± 0.02 | |
| NaCl | 0.42 ± 0.07 | 16.5 ± 0.4 | * | 0.43 ± 0.07 a | 0.39 ± 0.00 a | 0.38 ± 0.00 a | 0.43 ± 0.07 a | 0.22 ± 0.00 | 0.25 ± 0.03 | |
| Total phenolic content and antioxidant activity | ||||||||||
| TPC (mg GAE/g) | 3.02 ± 0.40 | 1.27 ± 0.40 | * | 1.06 ± 0.05 a | 1.10 ± 0.20 a | 0.73 ± 0.03 b | 0.68 ± 0.04 b | 0.43 ± 0.02 | 0.39 ± 0.02 | |
| FRAP (mg FSE/g) | 2.88 ± 0.23 | 0.86 ± 0.20 | * | 3.68 ± 0.46 b | 4.15 ± 0.32 a | 4.17 ± 0.12 a | 4.50 ± 0.13 a | 2.44 ± 0.07 | 2.56 ± 0.07 | |
| Amino Acids | SPI | HIM | RP | CP | RPA | CPA | CP | CPA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Weight | Dry Weight | Fresh Weight | ||||||||
| Non-essential | ||||||||||
| Asp | 64 ± 2 | 11 ± 0 | * | 6.7 ± 0.3 b | 6.5 ± 0.2 b | 7.6 ± 0.3 a | 7.9 ± 0.2 a | 3.8 ± 0.1 | 4.5 ± 0.1 | * |
| Glu | 86 ± 3 | 22 ± 0 | * | 34 ± 2 b | 33 ± 1 b | 38 ± 2 a | 40 ± 1 a | 19.6 ± 0.8 | 22.7 ± 0.8 | * |
| Ser | 32 ± 1 | 5.1 ± 0.1 | * | 6.4 ± 0.4 bc | 6.1 ± 0.2 c | 7.0 ± 0.3 ab | 7.3 ± 0.3 a | 3.6 ± 0.1 | 4.1 ± 0.2 | * |
| Gly | 32 ± 1 | 4.4 ± 0.1 | * | 4.1 ± 0.6 a | 3.8 ± 0.1 a | 4.5 ± 0.2 a | 4.6 ± 0.2 a | 2.2 ± 0.1 | 2.6 ± 0.1 | * |
| Arg | 50 ± 2 | 5.4 ± 0.2 | * | 5.6 ± 0.1 b | 5.9 ± 0. 3 b | 6.5 ± 0.3 a | 6.8 ± 0.3 a | 3.4 ± 0.2 | 3.9 ± 0.2 | * |
| Ala | 50 ± 2 | 7.1 ± 0.2 | * | 4.3 ± 0.2 b | 4.2 ± 0.2 b | 5.1 ± 0.2 a | 5.3 ± 0.2 a | 2.4 ± 0.1 | 3.0 ± 0.1 | * |
| Tyr | 27 ± 1 | 2.1 ± 0.1 | * | 1.8 ± 0.1 b | 1.9 ± 0.1 b | 2.4 ± 0.2 a | 2.5 ± 0.1 a | 1.13 ± 0.05 | 1.44 ± 0.06 | * |
| Hyp | 0.126 ± 0.005 | 0.30 ± 0.01 | * | 0.126 ± 0.007 a | 0.119 ± 0.003 a | 0.131 ± 0.006 a | 0.126 ± 0.004 a | 0.069 ± 0.002 | 0.072 ± 0.002 | |
| Pro | 25.1 ± 0.8 | 3.9 ± 0.1 | * | 12.0 ± 0.4 b | 12.1 ± 0.6 b | 13.6 ± 0.5 a | 14.3 ± 0.4 a | 7.1 ± 0.3 | 8.2 ± 0.2 | * |
| Essential | ||||||||||
| Val | 34 ± 1 | 4.7 ± 0.1 | * | 4.6 ± 0.2 b | 4.6 ± 0.2 b | 5.3 ± 0.2 a | 5.6 ± 0.2 a | 2.7 ± 0.1 | 3.2 ± 0.1 | * |
| Met | 11 ± 1 | 2.3 ± 0.1 | * | 1.96 ± 0.09 b | 1.91 ± 0.08 b | 2.2 ± 0.1 a | 2.3 ± 0.1 a | 1.12 ± 0.05 | 1.30 ± 0.05 | * |
| Trp | 3.6 ± 0.2 | 0.9 ± 0.1 | * | 0.91 ± 0.02 ab | 0.99 ± 0.07 a | 0.79 ± 0.04 c | 0.88 ± 0.01 bc | 0.58 ± 0.04 | 0.500 ± 0.006 | * |
| Phe | 23 ± 1 | 3.4 ± 0.2 | * | 4.7 ± 0.1 b | 5.1 ± 0.2 b | 5.8 ± 0.3 a | 6.0 ± 0.2 a | 3.0 ± 0.1 | 3.4 ± 0.1 | * |
| Ile | 34 ± 2 | 4.4 ± 0.1 | * | 4.2 ± 0.3 bc | 3.8 ± 0.2 c | 4.6 ± 0.2 ab | 5.0 ± 0.2 a | 2.3 ± 0.1 | 2.8 ± 0.1 | * |
| Leu | 51 ± 2 | 6.6 ± 0.2 | * | 7.6 ± 0.4 b | 7.6 ± 0.3 b | 8.7 ± 0.4 a | 9.2 ± 0.3 a | 4.4 ± 0.2 | 5.3 ± 0.2 | * |
| Lys | 37 ± 1 | 4.5 ± 0.1 | * | 2.9 ± 0.6 a | 2.5 ± 0.1 a | 2.8 ± 0.1 a | 3.1 ± 0.2 a | 1.44 ± 0.05 | 1.75 ± 0.09 | * |
| Thr | 35 ± 1 | 5.5 ± 0.2 | * | 4.2 ± 0.3 bc | 3.8 ± 0.2 c | 4.4 ± 0.2 b | 4.7 ± 0.2 a | 2.2 ± 0.1 | 2.7 ± 0.1 | * |
| His | 15.7 ± 0.2 | 10.4 ± 0.2 | * | 3.3 ± 0.1 b | 3.2 ± 0.1 b | 3.8 ± 0.1 a | 3.8 ± 0.2 a | 1.9 ± 0.1 | 2.1 ± 0.1 | * |
| Total amino acids sum (%) | 61.2 | 10.4 | 10.9 | 10.7 | 12.3 | 12.9 | 6.3 | 7.4 | ||
| Amino Acids | SPI | HIM | RP | CP | RPA | CPA | CP | CPA | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dry Weight | Dry Weight | Fresh Weight | ||||||||
| Non-essential | ||||||||||
| Asp | 325 ± 10 | 1171 ± 33 | * | 263 ± 1 a | 227 ± 6 b | 167 ± 0.8 c | 125 ± 1 d | 133 ± 3 | 71 ± 1 | * |
| Glu | 4323 ± 129 | 2671 ± 33 | * | 141 ± 1 a | 112 ± 4 b | 141 ± 1 a | 100 ± 1 c | 65 ± 2 | 57 ± 1 | * |
| Asn | 131 ± 5 | 89 ± 3 | * | 217 ± 3 a | 209 ± 7 ab | 199 ± 0 b | 208 ± 3 ab | 122 ± 4 | 118 ± 2 | |
| Ser | 186 ± 3 | 145 ± 4 | * | 55 ± 2 c | 59 ± 1 c | 97 ± 2 a | 74 ± 0 b | 35 ± 1 | 42 ± 0 | * |
| Gln | 73 ± 4 | 894 ± 29 | * | 21 ± 2 c | 18 ± 0 c | 37 ± 1 a | 27 ± 1 b | 11 ± 0 | 15 ± 0 | * |
| Gly | 206 ± 10 | 185 ± 5 | 40 ± 2 c | 41 ± 3 c | 67 ± 1 a | 59 ± 1 b | 24 ± 2 | 34 ± 1 | * | |
| Arg | 338 ± 23 | 43 ± 2 | * | 89 ± 2 b | 94 ± 2 ab | 99 ± 2 a | 87 ± 2 b | 55 ± 1 | 49 ± 1 | * |
| Ala | 1195 ± 63 | 1016 ± 30 | * | 119 ± 3 c | 101 ± 2 d | 233 ± 2 a | 184 ± 4 b | 59 ± 1 | 105 ± 2 | * |
| Tyr | 262 ± 16 | 31 ± 1 | * | 43 ± 2 b | 38 ± 1 c | 53 ± 2 a | 39 ± 1 bc | 22 ± 1 | 22 ± 0 | |
| Hyp | 2.5 ± 0.1 | 3.3 ± 0.1 | * | 3.2 ± 0.1 a | 2.8 ± 0.2 b | 3.3 ± 0.1 a | 2.9 ± 0.1 ab | 1.6 ± 0.1 | 1.7 ± 1 | |
| Pro | 66 ± 0 | 56 ± 4 | 43 ± 0.1 c | 30 ± 1 d | 69 ± 3 a | 58 ± 2 b | 18 ± 1 | 33 ± 1 | * | |
| Essential | ||||||||||
| His | 111 ± 6 | 8613 ± 169 | * | 21 ± 1 c | 18 ± 2 c | 101 ± 3 b | 126 ± 2 a | 11 ± 1 | 71 ± 1 | * |
| Thr | 227 ± 7 | 69 ± 4 | * | 49 ± 1 c | 43 ± 0 d | 88 ± 1 a | 73 ± 2 b | 25 ± 0 | 41 ± 1 | * |
| Val | 188 ± 5 | 57 ± 1 | * | 37 ± 1 c | 32 ± 1 d | 62 ± 1 a | 51 ± 0 b | 19 ± 1 | 29 ± 0 | * |
| Met | 80 ± 7 | n.d. | 24 ± 0 a | 22 ± 1 a | 23 ± 1 a | 22 ± 1 a | 13 ± 0 | 12 ± 1 | ||
| Trp | 85 ± 3 | 16 ± 0 | * | 274 ± 3 a | 240 ± 6 b | 137 ± 2 c | 127 ± 0.3 c | 141 ± 4 | 72 ± 2 | * |
| Phe | 195 ± 14 | 71 ± 1 | * | 34 ± 1 c | 34 ± 2 c | 48 ± 1 a | 39 ± 1 b | 20 ± 1 | 22 ± 4 | |
| Ile | 213 ± 8 | 45 ± 1 | * | 31 ± 0 c | 24 ± 1 d | 52 ± 2 a | 39 ± 1 b | 14 ± 0 | 22 ± 5 | * |
| Leu | 273 ± 15 | 35 ± 2 | * | 44 ± 1 c | 37 ± 0 d | 80 ± 1 a | 65 ± 1 b | 22 ± 0 | 37 ± 6 | * |
| Lys | 498 ± 7 | 57 ± 4 | * | 31 ± 0 d | 45 ± 2 c | 93 ± 2 a | 79 ± 3 b | 27 ± 3 | 45 ± 2 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, B.C.C.; Machado, M.; Machado, S.; Costa, A.S.G.; Bessada, S.; Alves, R.C.; Oliveira, M.B.P.P. Algae Incorporation and Nutritional Improvement: The Case of a Whole-Wheat Pasta. Foods 2023, 12, 3039. https://doi.org/10.3390/foods12163039
Oliveira BCC, Machado M, Machado S, Costa ASG, Bessada S, Alves RC, Oliveira MBPP. Algae Incorporation and Nutritional Improvement: The Case of a Whole-Wheat Pasta. Foods. 2023; 12(16):3039. https://doi.org/10.3390/foods12163039
Chicago/Turabian StyleOliveira, Bárbara C. C., Marlene Machado, Susana Machado, Anabela S. G. Costa, Sílvia Bessada, Rita C. Alves, and Maria Beatriz P. P. Oliveira. 2023. "Algae Incorporation and Nutritional Improvement: The Case of a Whole-Wheat Pasta" Foods 12, no. 16: 3039. https://doi.org/10.3390/foods12163039