Algal Carotenoids: Chemistry, Sources, and Application
Abstract
:1. Introduction
2. Carotenoids
2.1. Chemistry
2.2. Extraction
2.3. Identification, Separation, and Quantification
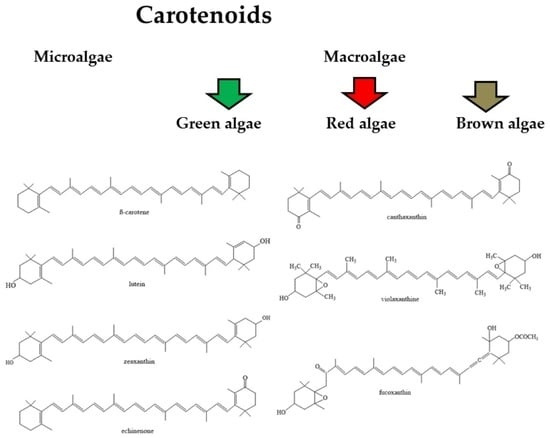
3. Algal Carotenoids
3.1. Carotenoids in Microalgae
| Species | Origin | Solvent | Extraction Method | Extraction Conditions | Detection Method | Result | Reference |
|---|---|---|---|---|---|---|---|
| Amphora sp. | - | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (1.21 mg/g) | [51] |
| Chaetoceros muelleri | North Pacific | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (2.92 mg/g) | [51] |
| Chlorella salina | India | MetOH | UAME | Sonication (35 kHz, 30 min, 40 °C) | HPLC-DAD | Lutein (2.92 mg/g) | [26] |
| Chlorella vulgaris | Czech Republic | Hep-EtOH-water, THF, DCM | CSE, UAE | CSE: maceration (30 min); UAE: sonication (38 kHz, 47.7707 W/cm, 10, 20 and 30 min, 25 °C) | HPLC-DAD | Lutein (0.22–3.20 mg/g) | [28] |
| Spain | EtOH | PEF | PEF pretreatment: temperature (10, 25 and 40 °C), distance between electrodes (0.25 cm), area (1.76 cm); maceration (dark); centrifugation | HPLC-DAD | Lutein (0.75 mg/g) | [50] | |
| Chlorococcum humicola | Thailand | Liquefied DME, MetOH, ACE | Liquefied DME extraction, CSE | DME: time (6–60 min); temperature (30–47 °C); CSE: magnetic stirring (400 rpm); time (6–60 min); temperature (30–47 °C) | UV/Vis, HPLC-DAD | Total carotenoids (4.14 mg/g) | [56] |
| India | EtOH | CSE | HPLC-UV/Vis | Violaxanthin (24.98 mg/g fw), astaxanthin (37.31 mg/g fw), lutein (48.36 mg/g fw), zeaxanthin (22.98 mg/g), α-carotene (32.90 mg/g), β-carotene (46.31 mg/g) | [57] | ||
| Chrysotila carterae | USA | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (1.04 mg/g) | [51] |
| Desmodesmus sp. F51 | Taiwan | - | Pressure application | High-pressure homogenization (10–40 kpsi, cycles 1 to 4) | UV/Vis, HPLC-UV/Vis | Total carotenoids (0.6–8.2 mg/g), neoxanthin, violaxanthin, lutein, α-carotene, β-carotene | [58] |
| Dunaliella salina | - | CO2 with EtOH/MetOH | SFE | Pressure (20 and 30 MPa); temperature (308.15, 318.15, and 328.15 K); co-solvent (5%) | UV/Vis | Total carotenoids (4–25 mg/g) | [47] |
| India | ACE | UAE | Vortexing (15 s); sonication (10 min); homogenization with solvent (4 days) | UV/Vis | Total carotenoids (3.2–13.9 µg/mL) | [48] | |
| Haematococcus pluvialis | Canada | ACE, MetOH, EtOH | UAE | Sonication (35% amplitude at 20 kHz, 5, 15, 25, and 35 min) | UV/Vis | Total astaxanthin (0.15–0.36 mg/g) | [27] |
| Czech Republic | EtOH | CSE | Vortexing (5 min) with glass beads; centrifugation (3000 rpm, 3 min) | HPLC-DAD, LC-QTOF-MS | Lutein (1.12 mg/g), β-carotene (0.89 mg/g), adonixanthin (0.17 mg/g), antheraxanthin (0.04 mg/g), neoxanthin (0.44 mg/g), astaxanthin (0.06 mg/g), echinenone (0.06 mg/g), total carotenoids (3.62 mg/g) | [59] | |
| Italy | ACE, EtOH, Hex, Chl/MetOH | PLE | Pressure (50 and 100 bar); temperature (20–100 °C); heating time (5 min); time (20 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | HPLC-DAD | Astaxanthin (3.96–30.02 µg/g) | [22] | |
| China | EtOAc | CSE, UAE, MAE, MFAE | CSE: maceration with stirring (230 rpm, 50 min, 50 °C); UAE: sonication (100 W, 60 min, 40 °C); MAE: (30 min; 45 °C); MFAE: (field 20 mT; 50 MHz; 60 min, RT) | HPLC-DAD | Astaxanthin (76.5–111.2 mg/g) | [21] | |
| Brazil | DCM | Enzymatic lysis (β-1,3-glucanase, xylanes, and protease) | Enzymatic lysis in combination with ultrasonication (40 kHz) | UV/Vis | Total carotenoids (0.50–1.25 mg/g) | [23] | |
| Isochrysis galbana | - | SFE | Pressure (30 MPa); temperature (50 °C), co-solvent (4% ethanol); CO2 flow rate (7.2 g/min for 120 min) | UV/Vis | Fucoxanthin (7.5 mg/g) | [60] | |
| Navicula sp. | - | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (1.49 mg/g) | [51] |
| Nanofrustulum shiloi | Turkey | EtOH | UAE | Ultrasonic bath, 50 °C, 15 min | HPLC-DAD | Fucoxanthin (19.75–38.06 mg/g) | [61] |
| Pheodactylum tricornutum | United Kingdom | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (1.87 mg/g) | [51] |
| Tisochrysis lutea | Tahiti | ACE | PLE | Pressure (1500 and 2000 psi); heating time (5 min); flushing solvent volume (6.6 mL); nitrogen purging (1 min) | UV/Vis, HPLC-DAD | Fucoxanthin (2.05 mg/g) | [51] |
3.2. Carotenoids in Green Algae (Chlorophyta)
| Species | Origin | Solvent | Extraction Method | Extraction Conditions | Detection Method | Result | Reference |
|---|---|---|---|---|---|---|---|
| Bryopsis sp. | India | ACE | CSE | Maceration | HPLC-DAD | Fucoxanthin (3.44 µg/g), neoxanthin (2.11 µg/g), violaxanthin (0.84 µg/g), lutein (4.06 µg/g), zeaxanthin (1.62 µg/g) | [65] |
| Caulerpa lentillifera | Indonesia | - | - | - | UV/Vis | Total carotenoids (1.31–2.29 µg/g) | [74] |
| Malaysia | ACE, Hex | CSE | Mixing; centrifugation | UV/Vis, HPLC-DAD | Total carotenoids (63.5 µg/g), zeaxanthin (21.30 µg/g), lutein (21.13 µg/g), β-carotene (10.7 µg/g), violaxanthin (8.93 µg/g) | [68] | |
| Malaysia | EtOH | CSE | Stirring (24 h, RT) | HPTLC, UHPLC-ESI/HRMS/MS | β-carotene (0.19 mg/g), astaxanthin (0.03 mg/g), canathaxanthin (0.15 mg/g), β-cryptoxanthin (0.013 mg/g), zeaxanthin (0.036 mg/g) | [69] | |
| Caulerpa racemose | India | ACE | CSE | Homogenization (24 h, dark, RT); centrifugation (5000 rpm, 15 min) | UV/Vis | Total carotenoids (0.04 mg/g) | [75] |
| Philippines | ACE | UAE | Sonication (pulse 2, amplitude 100, 2 min), maceration (dark, 4 °C, 24 h); centrifugation (36,000× g, 4 min) | HPLC-UV/Vis | β-carotene (17.26 mg/g) | [63] | |
| Indonesia | EtOH | CSE | Maceration (24 h, dark, RT, with stirring), sonication (40 °C, 30 min), filtration | UHPLC-ESI/HRMS/MS | β-carotene (0.06–0.21 mg/g), β-cryptoxanthin (0.02–0.07 mg/g), fucoxanthin (0.01–0.06 mg/g), astaxanthin (0.03–0.08 mg/g), canthaxanthin (0.04–0.16 mg/g), zeaxanthin (0.05–0.09 mg/g), lutein (0.02–0.06 mg/g) | [76] | |
| Cladophora rivularis | Poland | EtOH-water | Soxhlet, UAE, MAE, SFE | Soxhlet; sonication using ultrasonic bath; MAE (800 W, yield 100%); SFE (CO2, flow 10 mL/min; co-solvent: ethanol, flow 1 mL/min, dynamic mode (25 min), static mode (10 min), dynamic mode (25 min), pressure 350 bar) | UV/Vis | Total carotenoids: Soxhlet (0.9 µg/mL), UAE (0.6 µg/mL), MAE (1.0 µg/mL), SFE (0.3 µg/mL) | [77] |
| Caulerpa scalpelliformis | India | ACE | CSE | Homogenization (24 h, dark, RT); centrifugation (5000 rpm, 15 min) | UV/Vis | Total carotenoids (≈0.028 mg/g) | [75] |
| Caulerpa sertularioides | Mexico | ACE | CSE | Maceration; incubation (4 °C, 24 h); centrifugation (3200× g, 10 min, 4 °C) | HPLC-DAD | Siphoxanthin (3.64% fw), neoxanthin (3.66% fw), violaxanthin (8.05% fw), lutein (2.38% fw), siphonein (5.8% fw), α-carotene (1.13% fw), β-carotene (5.58% fw) | [66] |
| Cladophora glomerata | Lithuanian | EtOH, ACE | CSE | Shaking; centrifugation | UV/Vis | Total carotenoids (0.17–0.23 mg/g), lutein (0.11–0.17 mg/g) | [78] |
| Poland | EtOH-Water | Soxhlet, UAE, MAE, SFE | Soxhlet; sonication using ultrasonic bath; MAE (800 W, yield 100%); SFE (CO2, flow 10 mL/min; co-solvent: ethanol, flow 1 mL/min, dynamic mode (25 min), static mode (10 min), dynamic mode (25 min), pressure 350 bar) | UV/Vis | Total carotenoids: Soxhlet: 1.7 µg/mL; UAE: 0.5 µg/mL, MAE: 3.0 µg/mL, SFE: 1.0 µg/mL | [77] | |
| Cladophora sp. | India | ACE | CSE | Maceration | HPLC-DAD | Lutein (248.67 µg/g), zeaxanthin (50.20 µg/g) | [65] |
| Chaetomorpha antennina | India | ACE | CSE | Homogenization (24 h, dark, RT); centrifugation (5000 rpm, 15 min) | UV/Vis | Total carotenoids (≈0.027 mg/g) | [75] |
| India | ACE | CSE | Maceration | HPLC-DAD | Neoxanthin (2.11 µg/g), violaxanthin (0.84 µg/g), lutein (4.06 µg/g), zeaxanthin (1.62 µg/g) | [65] | |
| Chaetomorpha linum | Bulgaria | MetOH, Hex-DCM | CSE | Homogenization (3 min) | HPLC-UV/FLD | Astaxanthin (0.15 µg/g), β-carotene (0.17 µg/g) | [72] |
| Coelastrella sp. | Japan | Et2O-Chl-MetOH | CSE | Homogenization by glass beads; centrifugation | HPLC | Astaxanthin (31.5%), β-carotene (0.25%) | [79] |
| Codium cylindricum | Japan | Hex-ACE | CSE | Maceration with stirring (overnight, 4 °C) | HPLC-DAD | Siphonaxanthin (68% of the total lipid fraction) | [73] |
| Codium fragile | Indonesia | - | - | UV/Vis | Total carotenoids (4.28–6.05%) | [80] | |
| Enteromorpha intestinalis (Linnaeus) Nees | Turkey | ACE | - | - | UV/Vis | Total carotenoids (4.21 µg/g) | [81] |
| Enteromorpha intestinalis | Turkey | Water | CSE | Boiling (1 h) | UV/Vis | Total carotenoids (0.49 mg/g) | [64] |
| Halimeda opuntia (Linnaeus) Lamouroux | Malaysia | Chl-MetOH | CSE | Mixing (15 min); filtering; centrifugation (2000 rpm, 8 min) | UV/Vis | Total carotenoids (0.12 mg/g) | [82] |
| Monostroma nitidum | Japan | DME | - | Liquified DME extraction (vapor pressure 0.79 ± 0.02 MPa, flow 10 ± 1 mL/min, 33 min, 35 ± 1 °C) | HPLC | Lutein (0.30 mg/g) | [70] |
| Rhizoclonium riparium | Mexico | ACE | CSE | Maceration; incubation (4 °C, 24 h); centrifugation (3200× g, 10 min, 4 °C) | HPLC-DAD | Violaxanthin (6.12% fw), lutein (15.62% fw), dehydrolutein (2.50% fw), astaxanthin (1.52% fw), α-carotene (1.22% fw), β-carotene (1.86% fw) | [66] |
| Scenedesmus sp. | Turkey | EtOH-water (3:1 v/v), EtOAc, Hex, water | UAE | Sonication (20 min), stirring (1 h, RT), centrifugation (3800× g, 10 min) | UV/Vis | Total carotenoids (0.02–0.80 mg/g) | [83] |
| Trentepohlia abietina | India | ACE with BHT | CSE | Homogenization using mortar (dark); centrifugation; filtration | HPLC-UV/Vis | β-cryptoxanthin (0.15–0.34 µg/g), lutein (0.003–0.006 µg/g), β-carotene (230–585 µg/g) | [84] |
| Trentepohlia arborum | India | ACE with BHT | CSE | Homogenization using mortar (dark); centrifugation; filtration | HPLC-UV/Vis | β-cryptoxanthin (0.006–0.625 µg/g), lutein (0.002–0.006 µg/g), β-carotene (291–606 µg/g) | [84] |
| Trentepohlia diffracta | India | ACE with BHT | CSE | Homogenization using mortar (dark); centrifugation; filtration | HPLC-UV/Vis | β-cryptoxanthin (0.47–2.31 µg/g), lutein (0.001–0.005 µg/g), β-carotene (331–974 µg/g) | [84] |
| Trentepohlia umbrina | India | ACE with BHT | CSE | Homogenization using mortar (dark); centrifugation; filtration | HPLC-UV/Vis | β-cryptoxanthin (0.007–0.069 µg/g), lutein (0.001–0.003 µg/g), β-carotene (149–520 µg/g) | [84] |
| Ulva fasciata | Sri Lanka | ACE | CSE | Homogenization using mortar; centrifugation (3000 rpm, 15 min) | UV/Vis | Total carotenoids (0.17 µg/g) | [85] |
| Philippines | ACE | UAE | Sonication (pulse 2, amplitude 100, 2 min), left in the dark (4 °C, 24 h); centrifugation (36000× g, 4 min) | HPLC-UV/Vis | β-carotene (0.72 mg/g) | [63] | |
| India | ACE | CSE | Maceration | HPLC-DAD | Neoxanthin (0.26 µg/g), lutein (0.90 µg/g), zeaxanthin (0.25 µg/g) | [65] | |
| Ulva flexuosa | Poland | EtOH-water | Soxhlet, UAE, MAE, SFE | Soxhlet; sonication using ultrasonic bath; MAE (800 W, yield 100%); SFE (CO2, flow 10 mL/min; co-solvent: ethanol, flow 1 mL/min, dynamic mode (25 min), static mode (10 min), dynamic mode (25 min), pressure 350 bar) | UV/Vis | Total carotenoids: Soxhlet (1.3 µg/mL), UAE: 2.2 µg/mL, MAE (2.1 µg/mL), SFE (0.9 µg/mL) | [77] |
| Ulva lactuca | India | ACE | CSE | Homogenization (24 h, dark, RT); centrifugation (5000 rpm, 15 min) | UV/Vis | Total carotenoids (≈0.024 mg/g) | [75] |
| Portugal | MetOH | UAE | Stirring (1 h); sonication; centrifugation (2935× g, 10 min) | UV/Vis | Total carotenoids (0.20 mg/g) | [62] | |
| Portugal | Water, EtOAc, EtOH | CSE | Magnetic stirring (12 h, 25 °C); centrifugation (4000 rpm, 15 min) | UV/Vis, HPLC-DAD | Total carotenoids (≈0.14–0.32 mg/g), neoxanthin, violaxanthin, astaxanthin, lutein | [67] | |
| Sri Lanka | ACE | CSE | Homogenization using mortar; centrifugation (3000 rpm, 15 min) | UV/Vis | Total carotenoids (0.17 µg/g) | [85] | |
| India | ACE | CSE | Maceration | HPLC-DAD | Neoxanthin (0.47–0.61 µg/g), violaxanthin (0.02–0.03 µg/g), lutein (21.13–23.54 µg/g), zeaxanthin (11.26–12.14 µg/g) | [65] | |
| Ulva ohnoi | Brazil | Liquid N2, MetOH | CSE | Maceration; incubation (1 h, dark); centrifugation (12000× g, 10 min) | UV/Vis | Total carotenoids (18.90–32.20 µg/g) | [86] |
| Ulva rigida | Turkey | Water | CSE | Boiling (1 h) | UV/Vis | Total carotenoids (0.41 mg/g) | [64] |
| Portugal | ACE | CSE | Maceration (24 h) | UHPLC-DAD-ESI-MS | Lutein (0.42–1.20 µg/mg) | [71] | |
| Ulva prolifera | China | ACE | CSE | Maceration (4 °C, 12 h); centrifugation (10,000 rpm, 20 min) | UV/Vis | Total carotenoids (2.80–4.72 µg/mL) | [87] |
| India | ACE | CSE | Maceration | HPLC-DAD | Fucoxanthin (0.69 µg/g), neoxanthin (8.84 µg/g), violaxanthin (3.66 µg/g), lutein (10.23 µg/g), zeaxanthin (9.47 µg/g) | [65] | |
| China | EtOH, PET | CSE | Maceration (60 °C, 3 min) | HPLC | Lutein (4.84–5.91 µg/g), β-carotene (0.26–1.10 µg/g) | [88] | |
| Valoniopsis pachynema | India | ACE | CSE | Homogenization (24 h, dark, RT); centrifugation (5000 rpm, 15 min) | UV/Vis | Total carotenoids (≈0.022 mg/g) | [75] |
3.3. Carotenoids in Brown Algae (Phaeophyta)
| Species | Origin | Solvent | Extraction Method | Extraction Conditions | Detection Method | Result | Reference |
|---|---|---|---|---|---|---|---|
| Ascophyllum nodosum | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (2 mg/g), β-carotene (0.05 mg/g), other carotenoids (1.2 mg of fucoxanthin equivalents/g) | [92] |
| Bifurcaria bifurcata | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (0.71 mg/g), β-carotene (0.08 mg/g), other carotenoids (1.52 mg of fucoxanthin equivalents/g) | [92] |
| Cystoseira barbata | Turkey | Water | CSE | Boiling (1 h) | UV/Vis | Total carotenoids (2.195 mg/g) | [64] |
| Bulgaria | MetOH, Hex-DCM | CSE | Homogenization (3 min); saponification (50 °C, 30 min); extracted twice in n-hexane | HPLC-UV/FLD | Astaxanthin (3.0 µg/g fw), β-carotene (55.7 µg/g fw) | [72] | |
| Cystoseira crinita | Bulgaria | MetOH, Hex-DCM | CSE | Homogenization (3 min); saponification (50 °C, 30 min); extracted twice in n-hexane | HPLC-UV/FLD | Astaxanthin (1.39 µg/g fw), β-carotene (18.8 µg/g fw) | [72] |
| Dictyota dentata | Indonesia | ACE-MetOH | CSE | Homogenization using mortar; vortexing; centrifugation; drying with N2 | UV/Vis, HPLC-DAD | Total carotenoids (4.06 mg/g), fucoxanthin (4.11 mg/g, 0.29 mg/g fw) β-carotene (0.78 mg/g, 0.08 mg/g fw) | [97] |
| Fucus spiralis | Spain | EtOH | UAE | Vortexing (30 s); UAE: sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (2.48 mg/g), β-carotene (0.08 mg/g), other carotenoids (1.41 mg of fucoxanthin equivalents/g) | [92] |
| Fucus vesiculosus | Portugal | ACE | CSE | Maceration (24 h) | UHPLC-DAD-ESI-MS | Lutein (0.03–0.18 µg/mg), β-carotene (0.24–0.55 µg/mg), fucoxanthin (0.78–1.79 µg/mg) | [71] |
| Himanthalia elongata | Ireland | Hex, Et2O, Chl | CSE | Maceration, filtration, centrifugation (9168× g, 15 min) | LC-ESI-MS | Fucoxanthin (18.6 mg/g) | [99] |
| Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (0.67 mg/g), β-carotene (0.01 mg/g), other carotenoids (0.33 mg of fucoxanthin equivalents/g) | [92] | |
| Laminaria ochroleuca | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (4.35 mg/g), β-carotene (0.03 mg/g), other carotenoids (0.48 mg of fucoxanthin equivalents/g) | [92] |
| Laminaria saccharina | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (9.54 mg/g), β-carotene (0.07 mg/g), other carotenoids (0.48 mg of fucoxanthin equivalents/g) | [92] |
| Padina australis | Indonesia | ACE-MetOH | CSE | Homogenization using mortar; vortexing; centrifugation; drying with N2 | UV/Vis, HPLC-DAD | Total carotenoids (3.56 mg/g), fucoxanthin (1.64 mg/g, 0.22 mg/g fw), β-carotene (0.35 mg/g, 0.08 mg/g fw) | [97] |
| Padina durvillaei | Mexico | ACE | CSE | Homogenization; incubation (24 h, 4 °C); centrifugation (3200× g, 10 min, 4 °C) | HPLC-DAD | Fucoxanthin (33.9% fw), violaxanthin (4.5% fw), 19-Hex-fucoxanthin (5.80% fw), astaxanthin (0.42% fw), β-carotene (4.16% fw) | [66] |
| Padina pavonica | Malaysia | ACE, Chl | CSE | Saponification; mixing; centrifugation | UV/Vis, HPLC-DAD | Total carotenoids (0.1 mg/g), zeaxanthin (10.87 µg/g), lutein (7.21 µg/g), β-carotene (9.14 µg/g) | [68] |
| Pelvetia canaliculata | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (2.07 mg/g), β-carotene (0.06 mg/g), other carotenoids (0.45 mg of fucoxanthin equivalents/g) | [92] |
| Saccharina japonica | South Korea | Supercritical CO2 | SFE | Temperature (40–50 °C), pressure (200–300 bar), mixing ratio (27–75%) | HPLC | Fucoxanthin (2.08 mg/g) | [95] |
| Sargassum crassifolium | Indonesia | ACE-MetOH | Homogenization using mortar; vortexing; centrifugation; drying with N2 | UV/Vis, HPLC-DAD | Total carotenoids (1.01 mg/g), fucoxanthin (0.75 mg/g), fucoxanthin (0.09 mg/g fw), β-carotene (0.31 mg/g fw, 0.07 mg/g) | [97] | |
| Sargassum fusiforme | China | Ethyl lactate | UAE | Maceration (2 h, dark); sonication (500 W, 20 kHz), centrifugation (9000× g, 10 min, 4 °C) | HPLC | Fucoxanthin (0.6 mg/g by ethyl lactate) | [93] |
| Sargassum miyabei | Me2CO, Hex | CSE | Homogenization using mortar; filtration; separation over Al2O3 | HPLC-DAD | In the thallus (fucoxanthin: 57.9%, zeaxanthin: 12.5%, violaxanthin: 4.9%, neoxanthin: 3.1%, β-carotene: 2.4%, α-carotene: 0.2%) In the phylloids (fucoxanthin: 63.2%, zeaxanthin: 10.8%, violaxanthin: 2.7%, neoxanthin: 7.7%, β-carotene: 3.6%) | [98] | |
| Sargassum muticum | Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (5.79 mg/g), β-carotene (0.06 mg/g), other carotenoids (0.94 mg of fucoxanthin equivalents/g) | [92] |
| Sargassum polycystum | Malaysia | ACE, EtOH, MetOH | CSE | Shaking (40 °C, 24 h), centrifugation (2072× g, 10 min); filtration, drying | HPLC | Fucoxanthin (0.28 mg/g) | [100] |
| Malaysia | EtOH | CSE | Stirring (24 h, RT), filtration | HPTLC, UHPLC-ESI/HRMS/MS | Astaxanthin (0.26 mg/g), canathaxanthin (0.22 mg/g), β-cryptoxanthin 0.06 mg/g), fucoxanthin: 27.40 mg/g), zeaxanthin (0.14 mg/g), lutein (0.12 mg/g) | [69] | |
| Sargassum sp. | Mexico | EtOH | UAE, shock wave-assisted extraction | UAE: sonication (40 kHz, 160 W, 30 min); shock wave-assisted extraction: (delay between waves 50 and 950 μs, wave rate 0.5 Hz, voltage 3 and 6 kV, duration 404 ± 8 ns and 196 ± 8 ns) | UV/Vis, HPLC-UV/Vis | Fucoxanthin (UV/Vis: 0.29–0.39 mg/g, HPLC: 0.29–0.41 mg/g) | [94] |
| Scytosiphon lomentaria | Turkey | Water | CSE | Boiling (1 h) | UV/Vis | Total carotenoids (0.794 mg/g) | [64] |
| Sphaerotrichia divaricata | Japan | Chl-MetOH | CSE | Precipitation from lipid fraction | HPLC-DAD | Fucoxanthin (1.15 mg/g) | [101] |
| Turbinaria conoides | Indonesia | ACE-MetOH | Homogenization using mortar; vortexing; centrifugation; drying with N2 | UV/Vis, HPLC-DAD | Total carotenoids (0.55 mg/g), fucoxanthin (0.43 mg/g, 0.13 mg/g fw) β-carotene (0.16 mg/g, 0.07 mg/g fw) | [97] | |
| Undaria pinnatifida | Japan | EtOH | SFE-CO2 | Pressure (4000 psi), temperature (40 °C), Time (150 min); CO2 flow rate (1 mL/min) | HPLC-UV/Vis | Fucoxanthin (22.09 mg/g) | [96] |
| Spain | EtOH | UAE | Vortexing (30 s); sonication (500 W, 55 min); centrifugation (8400 rpm, RT, 7 min) | HPLC-DAD | Fucoxanthin (6.15 mg/g), β-carotene (0.30 mg/g), other carotenoids (2.42 mg of fucoxanthin equivalents/g) | [92] | |
| Zonaria tournefortii | Portugal | MetOH | UAE | Stirring (1 h); sonication; centrifugation (2935× g, 10 min) | UV/Vis | Total carotenoids (2.98 mg/g) | [62] |
3.4. Carotenoids in Red Algae (Rhodophyta)
| Species | Origin | Solvent | Extraction Method | Extraction Conditions | Detection Method | Result | Reference |
|---|---|---|---|---|---|---|---|
| Ahnfeltia plicata | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (≈1.8 µg/g), zeaxanthin (≈1.7 µg/g), β-carotene (≈2.2 µg/g) | [104] |
| Amphiroa rigida | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (1.05 µg/g fw) | [106] |
| Asparagopsis taxiformis | Portugal | MetOH | UAE | Stirring (1 h); sonication; centrifugation (2935× g, 10 min) | UV/Vis | Total carotenoids (0.13 mg/g) | [62] |
| Ceramium ciliatum | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.32 µg/g fw) | [106] |
| Ceramium rubrum | Turkey | ACE | - | UV/Vis | Total carotenoids (2.14 µg/g) | [81] | |
| Ceramium sp. | India | ACE | CSE | Maceration | HPLC-DAD | Fucoxanthin (4.85 µg/g), lutein (3.26 µg/g), zeaxanthin (0.66 µg/g) | [65] |
| Chondrus crispus | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.12 µg/g fw) | [106] |
| Portugal | MetOH | UAE | Stirring (1 h); sonication; centrifugation (2935× g, 10 min) | UV/Vis | Total carotenoids (0.21 mg/g) | [62] | |
| Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (≈3.0 µg/g), zeaxanthin (≈2.6 µg/g), β-carotene (≈7.8 µg/g) | [104] | |
| Corallina mediterranea | Egypt | ACE | CSE | Maceration (72 h, RT, dark) with intermittent shaking | UV/Vis | Total carotenoids (≈0.02 mg/g fw) | [107] |
| Delesseria sanguinea | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (≈3.7 µg/g), zeaxanthin (0.9 µg/g), β-carotene (≈4.2 µg/g) | [104] |
| Dilsea carnosa | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (≈7.0 µg/g), β-carotene (≈3.8 µg/g) | [104] |
| Ellisolandia elongata | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.89 µg/g fw) | [106] |
| Eucheuma denticulatum | Malaysia | EtOH | CSE | Stirring (24 h, RT) | HPTLC, UHPLC-ESI/HRMS/MS | β-carotene (0.047 mg/g), astaxanthin (0.03 mg/g), β-cryptoxanthin 0.036 mg/g), zeaxanthin (0.21 mg/g), lutein (0.88 mg/g), fucoxanthin (0.04 mg/g) | [69] |
| Malaysia | ACE, Hex | CSE | Mixing; centrifugation | UV/Vis, HPLC-DAD | Total carotenoids (33 µg/g), zeaxanthin (3.61 µg/g), lutein (9.57 µg/g), β-carotene (2.44 µg/g) | [68] | |
| Furcellaria lumbricalis | Denmark | ACN | CSE | Homogenization using mortar | HPLC-DAD | Lutein (13 µg/g), β-carotene (≈0.008 µg/g) | [104] |
| Gelidium crinale | Bulgaria | MetOH, Hex-DCM | CSE | Homogenization (3 min) | HPLC-UV/FLD | Astaxanthin (2.0 µg/g), β-carotene (33.8 µg/g) | [72] |
| Gelidium pusillum | Bangladesh | ACE | CSE | Incubation with shaking (250 rpm, 90 min, 20 °C); centrifugation (3000 rpm, 15 min) | UV/Vis | Total carotenoids (52.7 mg/g) | [103] |
| Gigartina acicularis | Turkey | Water | CSE | Boiling (1 h) | UV/Vis | Total carotenoids (0.59 µg/g) | [64] |
| Gracilaria changii | Malaysia | Hex-ACE-EtOH | CSE | Shaking; centrifugation (3000 rpm, 5 min, 4 °C) | UV/Vis | Total carotenoids (7.34 mg β-carotene equivalent/g) | [108] |
| Indonesia | ACE | CSE | Maceration (24 h, dark) | UV/Vis | Total carotenoids (0.24 µg/g) | [109] | |
| Gracilaria corticata | India | ACE | CSE | Incubation (45 min, dark); centrifugation (10,000× g, 5 min) | UV/Vis | Total carotenoids (12.82 µg/g) | [110] |
| India | ACE | CSE | Maceration | HPLC-DAD | Fucoxanthin (6.06 µg/g), lutein (0.26 µg/g), zeaxanthin (0.65 µg/g) | [65] | |
| Gracilaria edulis | India | ACE | CSE | Incubation (dark, 45 min); centrifugation (10,000× g, 5 min) | UV/Vis | Total carotenoids (2.99 µg/g) | [110] |
| Gracilaria tikvahiae | Malaysia | ACE, Hex | CSE | Mixing; centrifugation | UV/Vis, HPLC-DAD | Total carotenoids (25.1 µg/g), zeaxanthin (4.15 µg/g), lutein (8.86 µg/g), β-carotene (3.05 µg/g) | [68] |
| Gracilaria vermicu-lophylla | Denmark | ACN | CSE | Homogenization using mortar | HPLC-DAD | Zeaxanthin (0.93 µg/g), β-carotene (≈8.8 µg/g) | [104] |
| Mexico | ACE | CSE | Maceration; incubation (24 h, 4 °C); centrifugation (3200× g, 10 min, 4 °C) | HPLC-DAD | Zeaxanthin (7.83% fw), β-carotene (6.93% fw) | [66] | |
| Grateloupia filicina | India | ACE | CSE | Maceration | HPLC-DAD | Fucoxanthin (3.45 µg/g), neoxanthin (2.04 µg/g), violaxanthin (20.65 µg/g), lutein (18.38 µg/g), zeaxanthin (2.16 µg/g) | [65] |
| Grateloupia sp. | India | ACE | CSE | Maceration | HPLC-DAD | Lutein (166.58 µg/g), zeaxanthin (36.34 µg/g) | [65] |
| Halymenia durvillei | Indonesia | Hex, ACE, EtOH | CSE | Incubation (overnight, RT, dark) | UV/Vis | Total carotenoids (2.64–28.65 µg/g) | [111] |
| Hypnea musciformis | Bangladesh | ACE | CSE | Incubation with shaking (250 rpm, 90 min, 20 °C); centrifugation (3000 rpm,15 min) | UV/Vis | Total carotenoids (31.6 mg/g) | [103] |
| Jania rubens | Egypt | ACE | CSE | Maceration (72 h, RT, dark) with intermittent shaking | UV/Vis | Total carotenoids (≈0.02 mg/g fw) | [107] |
| Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (1.88 µg/g fw) | [106] | |
| Kappaphycus alvarezii | Brazil | Hex-ACE | UAE | Sonication (2.5 GHz, 1 h, dark); centrifugation (4000 rpm, 10 min) | UV/Vis, HPLC-UV/Vis | Lutein (112.02 µg/g), zeaxanthin (32.15 µg/g), α-carotene (13.36 µg/g), β-carotene (0.60 mg/g), β-carotene (0.24 mg/g) | [102] |
| Kappaphycus striatum | Malaysia | ACE, Hex | CSE | Mixing; centrifugation | UV/Vis, HPLC-DAD | Total carotenoids (57.0 µg/g), zeaxanthin (4.47 µg/g), lutein (38.60 µg/g), β-carotene (7.59 µg/g) | [68] |
| Liagora viscida | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (≈0.55 µg/g fw) | [106] |
| Nemalion elminthoides | Portugal | MetOH | UAE | Stirring (1 h); sonication; centrifugation (2935× g, 10 min) | UV/Vis | Total carotenoids (0.09 mg/g) | [62] |
| Neopyropia yezoensis | China | ACE, EtOAc, water | CSE | Vortexing (15 s); centrifugation (10,000× g, 5 min, 4 °C) | HPLC-DAD | Lutein (5.0 mg/g), β-carotene (0.6 mg/g), zeaxanthin (0.2 mg/g), α-carotene (0.14 mg/g) | [112] |
| Mesophyllum lichenoides | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.15 µg/g fw) | [106] |
| Odonthalia dentata | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (1.4 µg/g), zeaxanthin (≈4.0 µg/g), β-carotene (≈2.2 µg/g) | [104] |
| Osmundea pinnatifida | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (≈0.75 µg/g fw) | [106] |
| Palmaria palmata | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (≈1.9 µg/g), β-carotene (1.9 µg/g) | [104] |
| Phycodrys rubens | Denmark | ACN | CSE | Homogenization using mortar | HPLC-MS | Lutein (15.2 µg/g), zeaxanthin (≈3.2 µg/g), β-carotene (15.7 µg/g) | [104] |
| Plocamium cartilagineum | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.40 µg/g fw) | [106] |
| Porphyra umbilicalis | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (1.88 µg/g fw) | [106] |
| Pterocladia capillacea | Egypt | ACE | CSE | Maceration (72 h, RT, dark) with intermittent shaking | UV/Vis | Total carotenoids (0.092 mg/g) | [107] |
| Pyropia orbicularis | Chile | Hex-ACE-EtOH | CSE | Homogenization; centrifugation (2130× g, 15 min) | UV/Vis | Total carotenoids (58.6 µg/g) | [113] |
| Pyropia yezoensis | Japan | MetOH, Chl | CSE | Maceration (24 h, RT, dark) | LC-MS, 1H-NMR | Lutein (3.46 mg/g), zeaxanthin, α-carotene, β-carotene, α-cryptoxanthin, β-cryptoxanthin, lutein-5,6-epoxide, antheraxanthin | [105] |
| China | MetOH-ACE | CSE | Homogenization; maceration | HPLC-DAD | Lutein, zeaxanthin, α-carotene, β-carotene | [114] | |
| Spyridia filamentosa | Mexico | ACE | CSE | Maceration; incubation (24 h, 4 °C); centrifugation (3200× g, 10 min, 4 °C) | HPLC-DAD | Lutein (33.28% fw), dihydrolutein (3.70% fw), astaxanthin (0.43% fw), α-carotene (2.30% fw), β-carotene (9.62% fw) | [66] |
| Sphaerococcus coronopifolius | Portugal | ACE | CSE | Homogenization using mortar (10 min); stirring (30 min); centrifugation (12,500× g, 20 min, 4 °C) | UV/Vis | Total carotenoids (0.70 µg/g fw) | [106] |
| Spyridia filamentosa | Mexico | ACE | CSE | Homogenization using mortar | HPLC-DAD | Lutein (13 µg/g), β-carotene (≈0.008 µg/g) | [104] |
4. Potential Applications of Algal Carotenoids
5. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F.; Brown, J. Carotenoid Pigments in Seafoods and Aquaculture. Crit. Rev. Food Sci. Nutr. 1998, 38, 1–67. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Weber, F. Carotenoids. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Elsevier: Amsterdam, The Netherlands, 2016; pp. 101–123. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Food, A. Carotenoids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–14. [Google Scholar]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of Marine Carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Xie, C.; Hill, D.R.A.; Barrow, C.J.; Dunshea, F.R.; Martin, G.J.O.; Suleria, H.A.R. Bioaccessibility, Bioavailability and Bioactivities of Carotenoids in Microalgae: A Review. Food Rev. Int. 2023, 1–30. [Google Scholar] [CrossRef]
- Gross, J. Pigments in Vegetables; Springer US: Boston, MA, USA, 1991. [Google Scholar] [CrossRef]
- Fernandes, A.S.; do Nascimento, T.C.; Jacob-Lopes, E.; De Rosso, V.V.; Zepka, L.Q. Carotenoids—A Brief Overview on Its Structure, Biosynthesis, Synthesis, and Applications. In Progress in Carotenoid Research; IntechOpen: London, UK, 2018; pp. 1–16. [Google Scholar] [CrossRef] [Green Version]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 3164312. [Google Scholar] [CrossRef] [Green Version]
- Regal, P.; Lamas, A.; Fente, C.A.; Franco, C.M.; Cepeda, A. Analysis and Metabolomics of Carotenoids. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 189–222. [Google Scholar]
- Cheng, S.-H.; Khoo, H.E.; Kong, K.W.; Nagendra Prasad, K.; Galanakis, C.M. Extraction of Carotenoids and Applications. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 259–288. [Google Scholar]
- Shankar, U.; Lenka, S.K.; Ackland, M.L.; Callahan, D.L. Comparison of Different Solvent Extraction Compositions for the Analysis of Phytoplankton Pigments and Lipids Using a Rapid and Sensitive LC-MS Method. Res. Sq. 2022, preprint. [Google Scholar] [CrossRef]
- Varaprasad, D.; Raga Sudha, N.; Nazaneen Parveen, S.; Chandrasekhar, T. Effect of Various Solvents on Chlorophyll and Carotenoid Extraction in Green Algae: Chlamydomonas Reinhardtii and Chlorella Vulgaris. Ann. Plant Soil Res. 2019, 21, 341–345. [Google Scholar]
- Valcareggi Morcelli, A.; da Silva Andrade, W.; Frankenberg, C.L.C.; Rech, R.; Marcílio, N.R. Extraction of Chlorophylls and Carotenoids from Microalgae: COSMO-SAC-Assisted Solvent Screening. Chem. Eng. Technol. 2021, 44, 1227–1232. [Google Scholar] [CrossRef]
- Poojary, M.; Barba, F.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [Green Version]
- Nejadmansouri, M.; Golmakani, M.-T.; Famouri, M. Comparison of Different Methods for Carotenoid Extraction from Dunaliella Salina. Int. J. Nutr. Sci. 2021, 6, 208. [Google Scholar] [CrossRef]
- Papapostolou, H.; Kachrimanidou, V.; Alexandri, M.; Plessas, S.; Papadaki, A.; Kopsahelis, N. Natural Carotenoids: Recent Advances on Separation from Microbial Biomass and Methods of Analysis. Antioxidants 2023, 12, 1030. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative Study of the Extraction Methods for Recovery of Carotenoids from Algae: Extraction Kinetics and Effect of Different Extraction Parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Fu, L.; Zhu, H.; Zhang, B. Effect of Extraction and Drying Methods on Antioxidant Activity of Astaxanthin from Haematococcus Pluvialis. Food Bioprod. Process. 2016, 99, 197–203. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of Astaxanthin from Microalga Haematococcus Pluvialis in Red Phase by Using Generally Recognized as Safe Solvents and Accelerated Extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Machado, F.R.S.; Trevisol, T.C.; Boschetto, D.L.; Burkert, J.F.M.; Ferreira, S.R.S.; Oliveira, J.V.; Burkert, C.A.V. Technological Process for Cell Disruption, Extraction and Encapsulation of Astaxanthin from Haematococcus Pluvialis. J. Biotechnol. 2016, 218, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlčko, T.; Rathod, N.B.; Kulawik, P.; Ozogul, Y.; Ozogul, F. The Impact of Aromatic Plant-Derived Bioactive Compounds on Seafood Quality and Safety. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 275–339. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; Del Valle, J.M. Astaxanthin Extraction from Haematococcus pluvialis Using CO2-Expanded Ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, S.; Rajasree Radhika, S.R.; Suman, T.Y.; Aranganathan, L. Ultrasound-Assisted Microextraction of β, ε-Carotene-3, 3′-Diol (Lutein) from Marine Microalgae Chlorella salina: Effect of Different Extraction Parameters. Biomass Convers. Biorefinery 2018, 8, 791–797. [Google Scholar] [CrossRef]
- Haque, F.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Intensified Green Production of Astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of Lutein from the Green Microalgae Chlorella vulgaris by Integrated Use of a New Extraction Protocol and a Multi-Injection High Performance Counter-Current Chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, J.M.; Kim, S.; Yoon, M.J.; Park, K.S. Chemical Transformation of Astaxanthin from Haematococcus pluvialis Improves Its Antioxidative and Anti-Inflammatory Activities. ACS Omega 2020, 5, 19120–19130. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; FitzGerald, R.J.; Vila-Soler, A.; Mendiola, J.; Ibáñez, E.; Brunton, N.P. Comparison of Extraction Methods for Selected Carotenoids from Macroalgae and the Assessment of Their Seasonal/Spatial Variation. Innov. Food Sci. Emerg. Technol. 2016, 37, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.-H.; Rajesh banu, J.; Kumar, G. Technological Advances in the Production of Carotenoids and Their Applications—A Critical Review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Zhang, L.; Shao, P.; Wu, W.; Chen, Z.; Li, J.; Renard, C.M.G.C. An Overview of Carotenoid Extractions Using Green Solvents Assisted by Z-Isomerization. Trends Food Sci. Technol. 2022, 123, 145–160. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean Recovery of Antioxidant Compounds from Plant Foods, by-Products and Algae Assisted by Ultrasounds Processing. Modeling Approaches to Optimize Processing Conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Machmudah, S.; Goto, M. Methods for Extraction and Analysis of Carotenoids. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3367–3411. [Google Scholar] [CrossRef]
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75, C55–C61. [Google Scholar] [CrossRef]
- Varzakas, T.; Kiokias, S. HPLC Analysis and Determination of Carotenoid Pigments in Commercially Available Plant Extracts. Curr. Res. Nutr. Food Sci. J. 2016, 4, 1–14. [Google Scholar] [CrossRef]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids. In Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 259–296. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of Analysis (Extraction, Separation, Identification and Quantification) of Carotenoids from Natural Products. J. Ecosyst. Ecography 2016, 6, 1–19. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and Measurement of Carotenoids by UV/VIS Spectrophotometry. Curr. Protoc. Food Anal. Chem. 2001, F2-2. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A Rapid and Sensitive Method for Determination of Carotenoids in Plant Tissues by High Performance Liquid Chromatography. Plant Methods 2015, 11, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaliere, C.; Capriotti, A.; La Barbera, G.; Montone, C.; Piovesana, S.; Laganà, A. Liquid Chromatographic Strategies for Separation of Bioactive Compounds in Food Matrices. Molecules 2018, 23, 3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Hosokawa, M. Health Impact of Marine Carotenoids. J. Food Bioact. 2018, 1, 31–40. [Google Scholar] [CrossRef] [Green Version]
- De Jesus Raposo, M.F.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Carotenoids from Marine Microalgae: A Valuable Natural Source for the Prevention of Chronic Diseases. Mar. Drugs 2015, 13, 5128–5155. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Clemente, I.; Garcia-Vaquero, M. Carotenoids from Microalgae. In Carotenoids: Properties, Processing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 149–188. [Google Scholar]
- Tirado, D.F.; Calvo, L. The Hansen Theory to Choose the Best Cosolvent for Supercritical CO2 Extraction of Β-Carotene from Dunaliella salina. J. Supercrit. Fluids 2019, 145, 211–218. [Google Scholar] [CrossRef]
- Singh, P.; Baranwal, M.; Reddy, S.M. Antioxidant and Cytotoxic Activity of Carotenes Produced by Dunaliella salina under Stress. Pharm. Biol. 2016, 54, 2269–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Rathod, V.K. Ultrasound Assisted Extraction of β-Carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Luengo, E.; Martínez, J.M.; Bordetas, A.; Álvarez, I.; Raso, J. Influence of the Treatment Medium Temperature on Lutein Extraction Assisted by Pulsed Electric Fields from Chlorella vulgaris. Innov. Food Sci. Emerg. Technol. 2015, 29, 15–22. [Google Scholar] [CrossRef]
- Ishika, T.; Moheimani, N.R.; Bahri, P.A.; Laird, D.W.; Blair, S.; Parlevliet, D. Halo-Adapted Microalgae for Fucoxanthin Production: Effect of Incremental Increase in Salinity. Algal Res. 2017, 28, 66–73. [Google Scholar] [CrossRef]
- Seabra, L.M.J.; Pedrosa, L.F.C. Astaxanthin: Structural and Functional Aspects. Rev. Nutr. 2010, 23, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A Mechanistic Review on Its Biological Activities and Health Benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.B.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Nouralishahi, A.; Alizadehdakhel, A.; Golzary, A. Factors Affecting Production of Beta-Carotene from Dunaliella salina Microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- Eghbali Babadi, F.; Boonnoun, P.; Nootong, K.; Powtongsook, S.; Goto, M.; Shotipruk, A. Identification of Carotenoids and Chlorophylls from Green Algae Chlorococcum humicola and Extraction by Liquefied Dimethyl Ether. Food Bioprod. Process. 2020, 123, 296–303. [Google Scholar] [CrossRef]
- Sivathanu, B.; Palaniswamy, S. Purification and Characterization of Carotenoids from Green Algae Chlorococcum humicola by HPLC-NMR and LC-MS-APCI. Biomed. Prev. Nutr. 2012, 2, 276–282. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Jing, K.; Ng, I.S.; Chen, J.; Chang, J.S.; Lu, Y. Disruption of Thermo-Tolerant Desmodesmus sp. F51 in High Pressure Homogenization as a Prelude to Carotenoids Extraction. Biochem. Eng. J. 2016, 109, 243–251. [Google Scholar] [CrossRef]
- Grujić, V.J.; Todorović, B.; Ambrožič-Dolinšek, J.; Kranvogl, R.; Ciringer, T. Diversity and Content of Carotenoids and Other Pigments in the Transition from the Green to the Red Stage of Haematococcus pluvialis Microalgae Identified by HPLC-DAD and LC-QTOF-MS. Plants 2022, 11, 1026. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Rincón, B.; de los Ángeles Martín, M.; del Carmen Gutiérrez, M.; Salinas, F.; Medina, E.; Cerezal, P. Microalga Isochrysis galbana Biorefinery: Obtaining Fucoxanthin and Biogas after Supercritical Fluid Extraction. J. Appl. Phycol. 2022, 34, 1997–2014. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.B.; Demir, D.; Demirel, Z.; Aktürk, M.; Çopur, Ö.; Conk-Dalay, M. Manipulation in Culture Conditions of Nanofrustulum shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules 2023, 28, 1988. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Biochemical Composition, Nutritional Value, and Antioxidant Properties of Seven Seaweed Species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An Analysis of the Nutritional and Health Values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two Chlorophyta Collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef]
- AK, I.; Turker, G. Antioxidant Activity of Five Seaweed Extracts. New Knowl. J. Sci. 2018, 7, 149–155. [Google Scholar]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid Composition of Locally Found Seaweeds of Dakshina Kannada District in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; Nieves-Soto, M.; Manzano-Sarabia, M.M.; Hernández-Garibay, E.; Lizardi-Mendoza, J.; Burgos-Hernández, A.; Hurtado-Oliva, M.Á. Gross Chemical Composition, Fatty Acids, Sterols, and Pigments in Tropical Seaweed Species off Sinaloa, Mexico. Cienc. Mar. 2019, 45, 101–120. [Google Scholar] [CrossRef] [Green Version]
- Pappou, S.; Dardavila, M.M.; Savvidou, M.G.; Louli, V.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from Ulva lactuca. Appl. Sci. 2022, 12, 2117. [Google Scholar] [CrossRef]
- Othman, R.; Amin, N.A.; Sani, M.S.A.; Fadzillah, N.A.; Jamaludin, M.A. Carotenoid and Chlorophyll Profiles in Five Species of Malaysian Seaweed as Potential Halal Active Pharmaceutical Ingredient (API). Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 1610–1616. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; June Chelyn, L.; Vimala, S.; Mohd Fairulnizal, M.N.; Brownlee, I.A.; Amin, I. Carotenoid Composition and Antioxidant Potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Kanda, H.; Wahyudiono; MacHmudah, S.; Goto, M. Direct Extraction of Lutein from Wet Macroalgae by Liquefied Dimethyl Ether without Any Pretreatment. ACS Omega 2020, 5, 24005–24010. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva rigida, Gracilaria sp. and Fucus vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobreva, D.A.; Panayotova, V.Z.; Stancheva, R.S.; Stancheva, M. Simultaneous HPLC Determination of Fat Soluble Vitamins, Carotenoids and Cholesterol in Seaweed and Mussel Tissue. Bulg. Chem. Commun. 2017, 49, 112–117. [Google Scholar]
- Zheng, J.; Manabe, Y.; Sugawara, T. Siphonaxanthin, a Carotenoid from Green Algae Codium cylindricum, Protects Ob/Ob Mice Fed on a High-Fat Diet against Lipotoxicity by Ameliorating Somatic Stresses and Restoring Anti-Oxidative Capacity. Nutr. Res. 2020, 77, 29–42. [Google Scholar] [CrossRef]
- Syamsuddin, R.; Azis, H.Y.; Badraeni, R. Comparative Study on the Growth, Carotenoid, Fibre and Mineral Content of the Seaweed Caulerpa lentillifera Cultivated Indoors and in the Sea. IOP Conf. Ser. Earth Environ. Sci. 2019, 370, 012019. [Google Scholar] [CrossRef]
- Roy, S. Screening and Partial Characterization of Natural Antioxidants from Seaweeds Collected from, Rameshwaram Southeast Coast of India. J. Mar. Sci. Res. Oceanogr. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Ben Gunawan, W.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of Chlorophylls and Carotenoids from Freshwater Algae Using Different Extraction Methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Nutautaitė, M.; Racevičiūtė-Stupelienė, A.; Bliznikas, S.; Jonuškienė, I.; Karosienė, J.; Koreivienė, J.; Vilienė, V. Evaluation of Phenolic Compounds and Pigments in Freshwater Cladophora glomerata Biomass from Various Lithuanian Rivers as a Potential Future Raw Material for Biotechnology. Water 2022, 14, 1138. [Google Scholar] [CrossRef]
- Saito, M.; Watanabe, H.; Sasaki, M.; Ookubo, M.; Yarita, T.; Shiraiwa, M.; Asayama, M. Coproduction of Lipids and Carotenoids by the Novel Green Alga Coelastrella sp. Depending on Cultivation Conditions. Biotechnol. Rep. 2023, 37, e00769. [Google Scholar] [CrossRef]
- Sunaryo; Syamsuddin, R.; Azis, H.Y.; Nur, M. Growth and Carotenoid Content of The Green Seaweed Codium fragile on Different Depth. Int. J. Sci. Res. Publ. 2022, 12, 230–235. [Google Scholar] [CrossRef]
- Turker, G.; Ak, I. Assesment of Antioxidant Actvity of Enteromoprha intestinalis (Linnaeus) Assesment of Antioxidant Actvity of Enteromoprha intestinalis (Linnaeus) Nees and Ceramium rubrum C. Agardh. In Proceedings of the International Conference on Science and Technology ICONST 2018, Prizren, Kosovo, 5–9 September 2018; pp. 729–734. [Google Scholar]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.A.I.N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary Screening of Antioxidant and Cytotoxic Potential of Green Seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Akın, D.; Sönmez, Ç.; Öktem, A.; Yücel, M.; Öktem, H.A. Phenolic Compounds, Carotenoids, and Antioxidant Capacities of a Thermo-Tolerant Scenedesmus sp. (Chlorophyta) Extracted with Different Solvents. J. Appl. Phycol. 2019, 31, 1675–1683. [Google Scholar] [CrossRef]
- Kharkongor, D.; Ramanujam, P. Spatial and Temporal Variation of Carotenoids in Four Species of Trentepohlia (Trentepohliales, Chlorophyta). J. Bot. 2015, 2015, 201641. [Google Scholar] [CrossRef] [Green Version]
- Egodavitharana, D.I.; Manori Bambaranda, B.V.A.S.; Mudannayake, D.C. Phytochemical Composition of Two Green Seaweeds (Ulva lactuca and Ulva fasciata) and Their Utilization as a Functional Ingredient in Crackers. J. Aquat. Food Prod. Technol. 2023, 32, 158–174. [Google Scholar] [CrossRef]
- Rocha, J.S.; Santos, D.; Candia, E.W.d.S.C.d.; Hayashi, L.; Bauer, C.M.; Maraschin, M.; Vieira, F.d.N. Composition Characterization of Ulva ohnoi Cultivated in a Biofloc System. Res. Sq. 2022, 1–22. [Google Scholar] [CrossRef]
- He, Y.; Ma, Y.; Du, Y.; Shen, S. Differential Gene Expression for Carotenoid Biosynthesis in a Green Alga Ulva prolifera Based on Transcriptome Analysis. BMC Genom. 2018, 19, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Li, M.; Wang, Y.; Shen, S. The R2R3-MYB Transcription Factor MYB44 Modulates Carotenoid Biosynthesis in Ulva prolifera. Algal Res. 2022, 62, 102578. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment Composition of Nine Brown Algae from the Iberian Northwestern Coastline: Influence of the Extraction Solvent. Mar. Drugs 2022, 20, 113. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, A.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical Characteristics of the Brown Seaweed Carotenoid Fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Garcia-Perez, P.; Carreira-Casais, A.; Silva, A.; Simal-Gandara, J.; Prieto, M.A. A HPLC-DAD Method for Identifying and Estimating the Content of Fucoxanthin, Β-carotene and Chlorophyll a in Brown Algal Extracts. Food Chem. Adv. 2022, 1, 100095. [Google Scholar] [CrossRef]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and Kinetic Modeling of Ultrasonic-Assisted Extraction of Fucoxanthin from Edible Brown Algae Sargassum fusiforme Using Green Solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.A.; González-Reyna, M.A.; Loske, A.M.; Fernández, F.; Torres-Ortiz, D.A.; Estevez, M. Weak Shock Wave-Mediated Fucoxanthin Extraction from Sargassum spp. and Its Electrochemical Quantification. Algal Res. 2022, 68, 102891. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent Extraction of Oil from Roasted Coffee (Coffea arabica) and Fucoxanthin from Brown Seaweed (Saccharina japonica) Using Supercritical Carbon Dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Yin, S.; Niu, L.; Shibata, M.; Liu, Y.; Hagiwara, T. Optimization of Fucoxanthin Extraction Obtained from Natural By-Products from Undaria pinnatifida Stem Using Supercritical CO2 Extraction Method. Front. Nutr. 2022, 9, 981176. [Google Scholar] [CrossRef] [PubMed]
- Heriyanto; Juliadiningtyas, A.D.; Shioi, Y.; Limantara, L.; Brotosudarmo, T.H.P. Analysis of Pigment Composition of Brown Seaweeds Collected from Panjang Island, Central Java, Indonesia. Philipp. J. Sci. 2017, 146, 323–330. [Google Scholar]
- Tabakaeva, O.V.; Tabakaev, A.V. Carotenoid Profile and Antiradical Properties of Brown Seaweed Sargassum miyabei Extracts. Chem. Nat. Compd. 2019, 55, 364–366. [Google Scholar] [CrossRef]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Characterization of Dietary Fucoxanthin from Himanthalia elongata Brown Seaweed. Food Res. Int. 2017, 99, 995–1001. [Google Scholar] [CrossRef]
- Shukor, M.I.; Darnis, D.S.; Sabarudin, N.S.; Normawaty, M.N.; Bakhtiar, M.T. Effects of Solvent Extraction and Drying Methods of Malaysian Seaweed, Sargassum polycystum on Fucoxanthin Content. AIP Conf. Proc. 2022, 2645, 030025. [Google Scholar]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from Edible Seaweed, Undaria pinnatifida, Shows Antiobesity Effect through UCP1 Expression in White Adipose Tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- La Macchia Pedra, A.G.; Ramlov, F.; Maraschin, M.; Hayashi, L. Cultivation of the Red Seaweed Kappaphycus alvarezii with Effluents from Shrimp Cultivation and Brown Seaweed Extract: Effects on Growth and Secondary Metabolism. Aquaculture 2017, 479, 297–303. [Google Scholar] [CrossRef]
- Hossain, M.S.; din Sifat, S.A.; Hossain, M.A.; Salleh, S.; Hossain, M.; Akter, S.; Hossain, M.B. Comparative Assessment of Bioactive Compounds, Antioxidant Capacity and Nutritional Quality of Red Seaweeds and Water Spinach. Reg. Stud. Mar. Sci. 2021, 46, 101878. [Google Scholar] [CrossRef]
- Parjikolaei, B.R.; Bruhn, A.; Eybye, K.L.; Larsen, M.M.; Rasmussen, M.B.; Christensen, K.V.; Fretté, X.C. Valuable Biomolecules from Nine North Atlantic Red Macroalgae: Amino Acids, Fatty Acids, Carotenoids, Minerals and Metals. Nat. Resour. 2016, 7, 157–183. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid Profiling of a Red Seaweed Pyropia yezoensis: Insights into Biosynthetic Pathways in the Order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.V.; Inácio, L.G.; Martins, M.; Afonso, C.; Pereira, L.; Mouga, T. Primary Composition and Pigments of 11 Red Seaweed Species from the Center of Portugal. J. Mar. Sci. Eng. 2022, 10, 1168. [Google Scholar] [CrossRef]
- Mohy El-Din, S.M.; El-Ahwany, A.M.D. Bioactivity and Phytochemical Constituents of Marine Red Seaweeds (Jania rubens, Corallina mediterranea and Pterocladia capillacea). J. Taibah Univ. Sci. 2016, 10, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant Activities and Polyphenolics of Various Solvent Extracts of Red Seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Arbit, N.I.S.; Omar, S.B.A.; Tuwo, A.; Soekendarsi, E. Effect of Global Warming Scenarios on Carotenoid Pigments Gracilaria changii. Int. J. Environ. Agric. Biotechnol. 2018, 3, 2039–2042. [Google Scholar] [CrossRef]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria edulis and Gracilaria corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanger, G.; Rarung, L.; Kaseger, B.; Timbowo, S. Composition of Pigments and Antioxidant Activity in Edible Red Seaweed Halimenia durvilae Obtained from North Sulawesi. Int. J. ChemTech Res. 2016, 10, 255–262. [Google Scholar]
- Zhao, W.; Hu, C.M.; Zhou, W.; Deng, Y.Y.; Xu, G.P.; Tian, C.C.; Lu, Q.Q.; Lu, S.; Zhang, M.R.; Yang, L.E. Carotenoids Participate in Adaptation/Resistance of Daily Desiccation in the Intertidal Red Alga Neopyropia yezoensis (Bangiales, Rhodophyta). Algal Res. 2022, 61, 102606. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; Heredia, V.; Pastén, A.; Di Scala, K. An Edible Red Seaweed (Pyropia orbicularis): Influence of Vacuum Drying on Physicochemical Composition, Bioactive Compounds, Antioxidant Capacity, and Pigments. J. Appl. Phycol. 2018, 30, 673–683. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Wang, L.; He, L.; Wang, G. High Light Intensity Increases the Concentrations of β-Carotene and Zeaxanthin in Marine Red Macroalgae. Algal Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative Effects of β-Carotene and Fucoxanthin on Retinol Deficiency Induced Oxidative Stress in Rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Kim, S.C.; Lee, J.-H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the Constituent of Laminaria japonica, Triggers AMPK-Mediated Cytoprotection and Autophagy in Hepatocytes under Oxidative Stress. BMC Complement. Altern. Med. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preuss, H.G.; Echard, B.; Yamashita, E.; Perricone, N.V. High Dose Astaxanthin Lowers Blood Pressure and Increases Insulin Sensitivity in Rats: Are These Effects Interdependent? Int. J. Med. Sci. 2011, 8, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Speranza, L.; Pesce, M.; Patruno, A.; Franceschelli, S.; de Lutiis, M.A.; Grilli, A.; Felaco, M. Astaxanthin Treatment Reduced Oxidative Induced Pro-Inflammatory Cytokines Secretion in U937: SHP-1 as a Novel Biological Target. Mar. Drugs 2012, 10, 890–899. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Therapeutic Effect of Seaweed Derived Xanthophyl Carotenoid on Obesity Management; Overview of the Last Decade. Int. J. Mol. Sci. 2020, 21, 2502. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, B.; Chauhan, O.P.; Mishra, A. Edible Seaweeds: A Potential Novel Source of Bioactive Metabolites and Nutraceuticals with Human Health Benefits. Front. Mar. Sci. 2021, 8, 740054. [Google Scholar] [CrossRef]
- Chao, P.C.; Hsu, C.C.; Liu, W.H. Renal Protective Effects of Porphyra dentate Aqueous Extract in Diabetic Mice. Biomedicine 2014, 4, 14–18. [Google Scholar] [CrossRef]
- Arunkumar, E.; Bhuvaneswari, S.; Anuradha, C.V. An Intervention Study in Obese Mice with Astaxanthin, a Marine Carotenoid—Effects on Insulin Signaling and pro-Inflammatory Cytokines. Food Funct. 2012, 3, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Heo, S.-J.; Kang, S.-M.; Ahn, G.; Jeon, Y.-J. Fucoxanthin Induces Apoptosis in Human Leukemia HL-60 Cells through a ROS-Mediated Bcl-XL Pathway. Toxicol. Vitr. 2010, 24, 1648–1654. [Google Scholar] [CrossRef]
- Polat, S.; Trif, M.; Rusu, A.; Šimat, V.; Čagalj, M.; Alak, G.; Meral, R.; Özogul, Y.; Polat, A.; Özogul, F. Recent Advances in Industrial Applications of Seaweeds. Crit. Rev. Food Sci. Nutr. 2021, 63, 2010646. [Google Scholar] [CrossRef]
- Bose, I.; Sujatha, T.; Singh, R.; Sunder, J.; Samaddar, G. Preliminary Study on Exploration of Seaweed for an Alternative to Antibiotic Growth Promoter in Poultry Feed Additives. agriRxiv 2021, 20210373201. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and Utilization of Seaweed Pigments in Food Processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Yusof, Z.; Khong, N.M.H.; Choo, W.S.; Foo, S.C. Opportunities for the Marine Carotenoid Value Chain from the Perspective of Fucoxanthin Degradation. Food Chem. 2022, 383, 132394. [Google Scholar] [CrossRef]
- Hamed, I.; Moradi, M.; Ezati, P.; O’Higgins, L.; Meléndez-Martínez, A.J.; Frleta Matas, R.; Šimat, V.; McClements, D.J.; Jakobsen, A.N.; Lerfall, J. Encapsulation of Microalgal-Based Carotenoids: Recent Advances in Stability and Food Applications. Trends Food Sci. Technol. 2023, 138, 382–398. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as Promising Resource of Bioactive Compounds: Overview of Novel Extraction Strategies and Design of Tailored Meat Products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s Bioactive Candidate Compounds to Food Industry and Global Food Security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Diprat, A.B.; Silveira Thys, R.C.; Rodrigues, E.; Rech, R. Chlorella Sorokiniana: A New Alternative Source of Carotenoids and Proteins for Gluten-Free Bread. LWT 2020, 134, 109974. [Google Scholar] [CrossRef]
- Lafarga, T.; Mayre, E.; Echeverria, G.; Viñas, I.; Villaró, S.; Acién-Fernández, F.G.; Castellari, M.; Aguiló-Aguayo, I. Potential of the Microalgae Nannochloropsis and Tetraselmis for Being Used as Innovative Ingredients in Baked Goods. LWT 2019, 115, 108439. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of Microalgae Incorporation on the Physicochemical, Nutritional, and Sensorial Properties of an Innovative Broccoli Soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Andrade, M.A.; Barbosa, C.H.; Souza, V.G.L.; Coelhoso, I.M.; Reboleira, J.; Bernardino, S.; Ganhão, R.; Mendes, S.; Fernando, A.L.; Vilarinho, F.; et al. Novel Active Food Packaging Films Based on Whey Protein Incorporated with Seaweed Extract: Development, Characterization, and Application in Fresh Poultry Meat. Coatings 2021, 11, 229. [Google Scholar] [CrossRef]
- Sáez, M.I.; Suárez, M.D.; Alarcón, F.J.; Martínez, T.F. Assessing the Potential of Algae Extracts for Extending the Shelf Life of Rainbow Trout (Oncorhynchus mykiss) Fillets. Foods 2021, 10, 910. [Google Scholar] [CrossRef]
- Pereira, A.G.; Otero, P.; Echave, J.; Carreira-Casais, A.; Chamorro, F.; Collazo, N.; Jaboui, A.; Lourenço-Lopes, C.; Simal-Gandara, J.; Prieto, M.A. Xanthophylls from the Sea: Algae as Source of Bioactive Carotenoids. Mar. Drugs 2021, 19, 188. [Google Scholar] [CrossRef]
- Pangestuti, R.; Siahaan, E.A. Seaweed-Derived Carotenoids; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Zaheer, K. Hen Egg Carotenoids (Lutein and Zeaxanthin) and Nutritional Impacts on Human Health: A Review. CyTA J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef] [Green Version]
- AftabUddin, S.; Siddique, M.A.M.; Habib, A.; Akter, S.; Hossen, S.; Tanchangya, P.; Abdullah Al, M. Effects of Seaweeds Extract on Growth, Survival, Antibacterial Activities, and Immune Responses of Penaeus monodon against Vibrio parahaemolyticus. Ital. J. Anim. Sci. 2021, 20, 243–255. [Google Scholar] [CrossRef]
- Abdel-Rahim, M.; Bahattab, O.; Nossir, F.; Al-Awthan, Y.; Khalil, R.H.; Mohamed, R. Dietary Supplementation of Brown Seaweed and/or Nucleotides Improved Shrimp Performance, Health Status and Cold-Tolerant Gene Expression of Juvenile Whiteleg Shrimp during the Winter Season. Mar. Drugs 2021, 19, 175. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red Seaweed (Asparagopsis taxiformis) Supplementation Reduces Enteric Methane by over 80 Percent in Beef Steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence That Oral Supplementation with Lycopene or Lutein Protects Human Skin against Ultraviolet Radiation: Results from a Double-Blinded, Placebo-Controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [Green Version]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Nurjannah, K.A.; Amaliah, N.A.; Junda, M.; Iriany, N.; Makkulawu, A.T.; Karim, H.; Azis, A.A.; Djawad, Y.A.; Jumadi, O. The Influence of Fermented Brown Algae Extract (Sargassum sp.) on Corn Plant Growth (Zea mays L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 911, 012051. [Google Scholar] [CrossRef]
- Baroud, S.; Tahrouch, S.; El Mehrach, K.; Sadki, I.; Fahmi, F.; Hatimi, A. Effect of Brown Algae on Germination, Growth and Biochemical Composition of Tomato Leaves (Solanum lycopersicum). J. Saudi Soc. Agric. Sci. 2021, 20, 337–343. [Google Scholar] [CrossRef]
- Widyastuti, S.; Geraldine, B.A.F.D.; Sunarwidhi, A.L.; Ariyana, M.D.; Prasedya, E.S.; Sunarpi, H. The Use of Brown Algae Extract to Extend Shelf Life and Improve Post Harvest Quality of Tomato Fruit. AIP Conf. Proc. 2019, 2199, 070008. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global Seaweed Farming and Processing in the Past 20 Years. Food Prod. Process. Nutr. 2022, 4, 23. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Generalić Mekinić, I.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal Carotenoids: Chemistry, Sources, and Application. Foods 2023, 12, 2768. https://doi.org/10.3390/foods12142768
Generalić Mekinić I, Šimat V, Rathod NB, Hamed I, Čagalj M. Algal Carotenoids: Chemistry, Sources, and Application. Foods. 2023; 12(14):2768. https://doi.org/10.3390/foods12142768
Chicago/Turabian StyleGeneralić Mekinić, Ivana, Vida Šimat, Nikheel Bhojraj Rathod, Imen Hamed, and Martina Čagalj. 2023. "Algal Carotenoids: Chemistry, Sources, and Application" Foods 12, no. 14: 2768. https://doi.org/10.3390/foods12142768