Salmonella spp., Escherichia coli and Enterobacteriaceae Control at a Pig Abattoir: Are We Missing Lairage Time Effect, Pig Skin, and Internal Carcass Surface Contamination?
Abstract
:1. Introduction
2. Materials and Methods
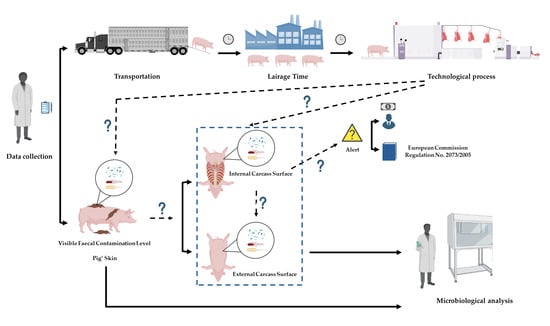
2.1. Data Collection
2.2. Sample Size and Sampling Procedures
2.3. Microbiological Analyses
2.3.1. Enterobacteriaceae and E. coli Counts
2.3.2. Isolation and Identification of E. coli O157
2.3.3. Isolation and Identification of Salmonella
2.4. Statistical Analysis
3. Results and Discussion
3.1. Enterobacteriaceae, E. coli Counts and Salmonella on Pig Skin
3.1.1. Influence of Pig Skin on Carcass Contamination
3.1.2. Influence of Visible Fecal Contamination Level
3.2. External Carcass Surface vs. Internal Carcass Surface
3.3. Effect of the Lairage Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD Publishing. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Fang, J.; Shen, Y.; Qu, D.; Han, J. Antimicrobial Resistance pro Fi Les and Characteristics of Integrons in Escherichia coli Strains Isolated from a Large-Scale Centralized Swine Slaughterhouse and Its Downstream Markets in Zhejiang, China. Food Control. 2019, 95, 215–222. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018, 16, 1–262. [Google Scholar] [CrossRef] [Green Version]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, L.G.; Júnior, A.B.; de Freitas Costa, E.; Duarte, A.S.R.; Albuquerque, E.R.; Kich, J.D.; Cardoso, M.; Nauta, M. Effect of Slaughterhouse and Day of Sample on the Probability of a Pig Carcass Being Salmonella-Positive According to the Enterobacteriaceae Count in the Largest Brazilian Pork Production Region. Int. J. Food Microbiol. 2016, 228, 58–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hoek, A.H.A.M.; de Jonge, R.; van Overbeek, W.M.; Bouw, E.; Pielaat, A.; Smid, J.H.; Malorny, B.; Junker, E.; Löfström, C.; Pedersen, K.; et al. A Quantitative Approach towards a Better Understanding of the Dynamics of Salmonella Spp. in a Pork Slaughter-Line. Int. J. Food Microbiol. 2012, 153, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Blagojevic, B.; Antic, D.; Ducic, M.; Buncic, S. Ratio between Carcass-and Skin-Microflora as an Abattoir Process Hygiene Indicator. Food Control. 2011, 22, 186–190. [Google Scholar] [CrossRef]
- Anonymous. Commission Regulation (EC No 2073/2005 of 15 November 2005 on Microbial Criteria for Foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. [Google Scholar]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae Counts on Pig and Ruminant Carcasses along the Slaughterline, Factors Influencing the Counts and Relationship between Visual Faecal Contamination of Carcasses and Counts: A review. EFSA Support. Publ. 2014, 11, 634E. [Google Scholar] [CrossRef] [Green Version]
- Lenahan, M.; Crowley, H.; Brien, S.B.O.; Byrne, C.; Sweeney, T.; Sheridan, J.J. The Potential Use of Chilling to Control the Growth of Enterobacteriaceae on Porcine Carcasses and the Incidence of E. coli O157: H7 in Pigs. J. Appl. Microbiol. 2009, 106, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 1–273. [Google Scholar] [CrossRef]
- DE KNEGT, L.V.; PIRES, S.M.; HALD, T. Attributing Foodborne Salmonellosis in Humans to Animal Reservoirs in the European Union Using a Multi-Country Stochastic Model. Epidemiol. Infect. 2015, 143, 1175–1186. [Google Scholar] [CrossRef]
- de Lima, E.d.S.C.; Pinto, P.S.d.A.; dos Santos, J.L.; Vanetti, M.C.D.; Bevilacqua, P.D.; de Almeida, L.P.; Pinto, M.S.; Dias, F.S. Isolamento de Salmonella Sp e Staphylococcus Aureus No Processo Do Abate Suíno Como Subsídio Ao Sistema de Análise de Perigos e Pontos Críticos de Controle—APPCC. Pesqui. Veterinária Bras. 2004, 24, 185–190. [Google Scholar] [CrossRef]
- Käsbohrer, A.; Protz, D.; Helmuth, R.; Nöckler, K.; Blaha, T.; Conraths, F.J.; Geue, L. Salmonella in Slaughter Pigs of German Origin: An Epidemiological Study. Eur. J. Epidemiol. 2000, 16, 141–146. [Google Scholar] [CrossRef]
- Vieira-Pinto, M.; Temudo, P.; Martins, C. Occurrence of Salmonella in the Ileum, Ileocolic Lymph Nodes, Tonsils, Mandibular Lymph Nodes and Carcasses of Pigs Slaughtered for Consumption. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.R.; Krautil, F.L.; Craven, J.A. Effect of Time in Lairage on Caecal and Carcass Salmonella Contamination of Slaughter Pigs. Epidemiol. Infect. 1987, 98, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 17604: 2003; Microbiology of Food and Animal Feeding Stuffs: Carcass Sampling for Microbiological Analysis. ISO: Geneve, Switzerland, 2003.
- ISO 21528-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Methods for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Method. ISO: Geneve, Switzerland, 2004.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase Positive Escherichia Coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl β-D-Glucuronide. ISO: Geneve, Switzerland, 2001.
- ISO 16654:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Escherichia coli O157. ISO: Geneve, Switzerland, 2001.
- Paton, A.W.; Paton, J.C. Detection and Characterization of Shiga Toxigenic Escherichia coli by Using Multiplex PCR Assays for Stx1, Stx2, EaeA, Enterohemorrhagic, E. coli HlyA, Rfb O111, and Rfb O157. J. Clin. Microbiol. 1998, 36, 598–602. [Google Scholar] [CrossRef] [Green Version]
- ISO 6579; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Salmonella Spp. ISO: Geneve, Switzerland, 2002.
- Walia, K.; Lynch, H.; Grant, J.; Duffy, G.; Leonard, F.C.; Lawlor, P.G.; Gardiner, G.E. The Efficacy of Disinfectant Misting in the Lairage of a Pig Abattoir to Reduce Salmonella and Enterobacteriaceae on Pigs Prior to Slaughter. Food Control. 2017, 75, 55–61. [Google Scholar] [CrossRef]
- Gill, C.O. Visible Contamination on Animals and Carcasses and the Microbiological Condition of Meat. J. Food Prot. 2004, 67, 413–419. [Google Scholar] [CrossRef]
- Rossel, R.; Jouffe, L.; Beloeil, P.A. Analysis of Factors Associated with Salmonella in a Pig Abattoir. Vet. Rec. 2009, 144, 655–661. [Google Scholar]
- Zdolec, N.; Kotsiri, A.; Houf, K.; Alvarez-Ordóñez, A.; Blagojevic, B.; Karabasil, N.; Salines, M.; Antic, D. Systematic Review and Meta-Analysis of the Efficacy of Interventions Applied during Primary Processing to Reduce Microbial Contamination on Pig Carcasses. Foods 2022, 11, 2110. [Google Scholar] [CrossRef]
- Salmela, S.P.; Fredriksson-Ahomaa, M.; Hatakka, M.; Nevas, M. Microbiological Contamination of Sheep Carcases in Finland by Excision and Swabbing Sampling. Food Control 2013, 31, 372–378. [Google Scholar] [CrossRef]
- Bouvet, J.; Bavai, C.; Rossel, R.; Le Roux, A.; Montet, M.P.; Ray-Gueniot, S.; Mazuy, C.; Arquillière, C.; Vernozy-Rozand, C. Prevalence of Verotoxin-Producing Escherichia coli and E. coli O157:H7 in Pig Carcasses from Three French Slaughterhouses. Int. J. Food Microbiol. 2001, 71, 249–255. [Google Scholar] [CrossRef]
- Bouvet, J.; Montet, M.P.; Rossel, R.; Le Roux, A.; Bavai, C.; Ray-Gueniot, S.; Mazuy, C.; Atrache, V.; Vernozy-Rozand, C. Effects of Slaughter Processes on Pig Carcass Contamination by Verotoxin-Producing Escherichia coli and E. coli O157:H7. Int. J. Food Microbiol. 2002, 77, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Park, H.J.; Jang, H.I.; Kim, S.A.; Imm, J.Y.; Hwang, I.G.; Rhee, M.S. Changes in Microbial Contamination Levels of Porcine Carcasses and Fresh Pork in Slaughterhouses, Processing Lines, Retail Outlets, and Local Markets by Commercial Distribution. Res. Vet. Sci. 2013, 94, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.S.; Jones, K. The Use of Selective and Differential Agars in the Isolation of Escherichia coli O157 from Dairy Herds. J. Appl. Bacteriol. 1996, 81, 663–668. [Google Scholar] [CrossRef]
- Müller, E.E.; Ehlers, M.M. Biolog Identification of Non-Sorbitol Fermenting Bacteria Isolated on E. coli O157 Selective CT-SMAC Agar. Water SA 2005, 31, 247–251. [Google Scholar] [CrossRef] [Green Version]
- da Silva, N.; Silveira, N.F.d.A.; Contreras, C.; Beraquet, N.J.; Yokoya, F.; Nascimento, C.A.D.; Oliveira, V.M.; Tse, C.L. Ocorrência de Escherichia coli O157: H7 em Produtos Cárneos e Sensibilidade dos Métodos de Detecção. Food Sci. Technol. 2001, 21, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-Typhoidal Salmonella in the Pig Production Chain: A Comprehensive Analysis of Its Impact on Human Health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Arguello, H.; Álvarez-Ordoñez, A.; Carvajal, A.; Rubio, P.; Prieto, M. Role of Slaughtering in Salmonella Spreading and Control in Pork Production. J. Food Prot. 2013, 76, 899–911. [Google Scholar] [CrossRef]
- Anonymous. Regulamento (CE) N° 853/2004 Do Parlamento Europeu e Do Conselho de 29 de Abril de 2004. J. União Eur. 2004, 139, 1–51. [Google Scholar]
- Belluco, S.; Barco, L.; Roccato, A.; Ricci, A. Variability of Escherichia coli and Enterobacteriaceae Counts on Pig Carcasses: A Systematic Review. Food Control. 2015, 55, 115–126. [Google Scholar] [CrossRef]
- Hauge, S.J.; Nafstad, O.; Skjerve, E.; Røtterud, O.J.; Nesbakken, T. Effects of Shearing and Fleece Cleanliness on Microbiological Contamination of Lamb Carcasses. Int. J. Food Microbiol. 2011, 150, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Dunne, G.; Lyng, J.; Bolton, D.J. The Development of a “clean Sheep Policy” in Compliance with the New Hygiene Regulation (EC) 853/2004 (Hygiene 2). Food Microbiol. 2007, 24, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Barco, L.; Belluco, S.; Roccato, A.; Ricci, A. Escherichia coli and Enterobacteriaceae Counts on Poultry Carcasses along the Slaughter Processing Line, Factors Influencing the Counts and Relationship between Visual Faecal Contamination of Carcasses and Counts: A Review. EFSA Support. Publ. 2017, 11, 1–111. [Google Scholar] [CrossRef] [Green Version]
- Vieira-Pinto, M.; Silva, M.M.; Esteves, A. Comparação Entre o Método Da Dupla Zaragatoa e o Da Excisão Na Determinação de Contagens de Microrganismos Totais Viáveis e de Enterobacteriaceae Na Superfície Interna e Externa de Carcaças de Suíno A Comparison between Double Swab and Excision Sampling M. Rev. Port. Ciências Veterinárias 2003, 98, 89–94. [Google Scholar]
- Morgan, I.R.; Krautil, F.; Craven, J.A. A Comparison of Swab and Maceration Methods for Bacterial Sampling of Pig Carcasses. J. Hyg. 1985, 95, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, C.; Baltzer, D.; Stephan, R. Microbiological Contamination of Cattle and Pig Carcasses at Five Abattoirs Determined by Swab Sampling in Accordance with EU Decision 2001/471/EC. Meat Sci. 2005, 69, 559–566. [Google Scholar] [CrossRef]
- Matsubara, E.N. Condição Higiênico-Sanitária de Meias-Carcaças de Suínos Após o Abate e Depois Do Resfriamento e Análise Da Utilização de Lista de Verificação Para Avaliar Boas Práticas No Abate de Suínos. 2005. Available online: https://teses.usp.br/teses/disponiveis/10/10134/tde-30102006-113224/publico/EstherNaomiMatsubara.pdf (accessed on 12 December 2020).
- Pearce, R.A.; Bolton, D.J. Excision vs Sponge Swabbing—A Comparison of Methods for the Microbiological Sampling of Beef, Pork and Lamb Carcasses. J. Appl. Microbiol. 2005, 98, 896–900. [Google Scholar] [CrossRef]
- Spescha, C.; Stephan, R.; Zweifel, C. Microbiological Contamination of Pig Carcases at Different Stages of Slaughter in Two Europian Union-Approved Abattoirs. J. Food Prot. 2006, 69, 2568–2575. [Google Scholar] [CrossRef]
- Lindblad, M. Microbiological Sampling of Swine Carcasses: A Comparison of Data Obtained by Swabbing with Medical Gauze and Data Collected Routinely by Excision at Swedish Abattoirs. Int. J. Food Microbiol. 2007, 118, 180–185. [Google Scholar] [CrossRef]
- Ghafir, Y.; Daube, G. Comparison of Swabbing and Destructive Methods for Microbiological Pig Carcass Sampling. Lett. Appl. Microbiol. 2008, 47, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.L.; MacDiarmid, S.; Cook, R. Salmonella, Escherichia coli O157:H7 and E. coli Biotype 1 in a Pilot Survey of Imported and New Zealand Pig Meats. Food Microbiol. 2009, 26, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, J.; Zheng, H.; Jin, X.; Shen, Y.; Lei, T.; Sun, X.; Pan, Z.; Jiao, X. Diversity of Salmonella Isolates and Their Distribution in a Pig Slaughterhouse in Huaian, China. Food Control. 2017, 78, 238–246. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 217/2014 of 7 March 2014 Amending Regulation (EC) No 2073/2005 as Regards Salmonella in Pig Carcases. Off. J. Eur. Union 2014, 69, 93–94. [Google Scholar]
- De Busser, E.V.; Maes, D.; Houf, K.; Dewulf, J.; Imberechts, H.; Bertrand, S.; De Zutter, L. Detection and Characterization of Salmonella in Lairage, on Pig Carcasses and Intestines in Five Slaughterhouses. Int. J. Food Microbiol. 2011, 145, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neves, E.; Antunes, P.; Tavares, A.; Themudo, P.; Cardoso, M.F.; Gärtner, F.; Costa, J.M.; Peixe, L. Salmonella Cross-Contamination in Swine Abattoirs in Portugal: Carcasses, Meat and Meat Handlers. Int. J. Food Microbiol. 2012, 157, 82–87. [Google Scholar] [CrossRef]
- Berends, B.R.; Van Knapen, F.; Snijders, J.M.A.; Mossel, D.A.A. Identification and Quantification of Risk Factors Spp. on Pork Carcasses. Int. J. Food Microbiol. 1997, 36, 199–206. [Google Scholar] [CrossRef]
- Hurd, S.; Mckean, J.D.; Griffith, R.W.; Wesley, I.V.; Rostagno, M.H.; Gailey, J.K.; Karriker, L.A. Lairage Is a Hazard for Salmonella Infection in Market Swine. Control 2002, 223–225. [Google Scholar]
- Duggan, S.J.; Mannion, C.; Prendergast, D.M.; Leonard, N.; Fanning, S.; Gonzales-Barron, U.; Egan, J.; Butler, F.; Duffy, G. Tracking the Salmonella Status of Pigs and Pork from Lairage through the Slaughter Process in the Republic of Ireland. J. Food Prot. 2010, 73, 2148–2160. [Google Scholar] [CrossRef]



| Primers a | Sequence (5′ 3′) | Amplicon Size b | Reference |
|---|---|---|---|
| stx1 (F) | ATA AAT CGC CAT TCG TTG ACT AC | 180 bp | [21] |
| stx1 (R) | AGA ACG CCC ACT GAG ATC ATC | ||
| stx2 (F) | GGC ACT GTC TGA AAC TGC TCC | 255 bp | |
| stx2 (R) | TCG CCA GTT ATC TGA CAT TCT G | ||
| O157(F) | CGG ACA TCC ATG TGA TAT GG | 259 bp | |
| O157(R) | TTG CCT ATG TAC AGC TAA TCC |
| Family/Species | PS (Log CFU/cm2) | ICS (Log CFU/cm2) | ECS (Log CFU/cm2) |
|---|---|---|---|
| Enterobacteriaceae | 3.27 ± 0.68 a | 1.65 ± 0.90 b | 0.29 ± 0.52 c |
| E. coli | 3.15 ± 0.63 a | 1.34 ± 0.89 b | 0.33 ± 0.58 c |
| E. coli O157 | ND | ND | ND |
| Salmonella spp. | 21% (21/100) | 9% (9/100) | 3% (3/100) |
| Pig | PS | ICS | ECS |
|---|---|---|---|
| 1 | Salmonella Rissen | - | - |
| 3 | Salmonella Rissen | - | - |
| 4 | Salmonella Rissen | - | - |
| 5 | Salmonella Rissen | - | - |
| 9 | Inconclusive a | - | - |
| 27 | - | - | Salmonella Rissen |
| 28 | - | Salmonella Rissen | - |
| 34 | - | - | Salmonella Rissen |
| 39 | Inconclusive a | - | - |
| 45 | Salmonella 1,4,[5],12:i:- | Salmonella Rissen | - |
| 46 | Salmonella 1,4,[5],12:i:- | - | - |
| 47 | - | Salmonella Rissen | - |
| 48 | Salmonella Rissen | - | - |
| 51 | Salmonella Rissen | - | - |
| 52 | Salmonella Rissen | - | - |
| 54 | Salmonella Rissen | - | - |
| 56 | Salmonella Derby | - | - |
| 57 | Salmonella Derby | - | - |
| 58 | Salmonella Derby | Salmonella Derby | - |
| 60 | - | - | Salmonella Derby |
| 86 | Salmonella 1,4,[5],12:i:- | - | - |
| 94 | Salmonella 1,4,[5],12:i:- | Salmonella1,4,[5],12:i:- | - |
| 95 | - | Salmonella 1,4,[5],12:i:- | - |
| 96 | Salmonella 1,4,[5],12:i:- | - | - |
| 97 | Salmonella 1,4,[5],12:i:- | - | - |
| 98 | - | Salmonella 1,4,[5],12:i:- | - |
| 99 | Salmonella 1,4,[5],12:i:- | Salmonella 1,4,[5],12:i:- | - |
| 100 | Salmonella 1,4,[5],12:i:- | Salmonella 1,4,[5],12:i:- | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias Costa, R.; Silva, V.; Leite, A.; Saraiva, M.; Lopes, T.T.; Themudo, P.; Campos, J.; Vieira-Pinto, M. Salmonella spp., Escherichia coli and Enterobacteriaceae Control at a Pig Abattoir: Are We Missing Lairage Time Effect, Pig Skin, and Internal Carcass Surface Contamination? Foods 2023, 12, 2910. https://doi.org/10.3390/foods12152910
Dias Costa R, Silva V, Leite A, Saraiva M, Lopes TT, Themudo P, Campos J, Vieira-Pinto M. Salmonella spp., Escherichia coli and Enterobacteriaceae Control at a Pig Abattoir: Are We Missing Lairage Time Effect, Pig Skin, and Internal Carcass Surface Contamination? Foods. 2023; 12(15):2910. https://doi.org/10.3390/foods12152910
Chicago/Turabian StyleDias Costa, Rui, Vanessa Silva, Ana Leite, Margarida Saraiva, Teresa Teixeira Lopes, Patrícia Themudo, Joana Campos, and Madalena Vieira-Pinto. 2023. "Salmonella spp., Escherichia coli and Enterobacteriaceae Control at a Pig Abattoir: Are We Missing Lairage Time Effect, Pig Skin, and Internal Carcass Surface Contamination?" Foods 12, no. 15: 2910. https://doi.org/10.3390/foods12152910