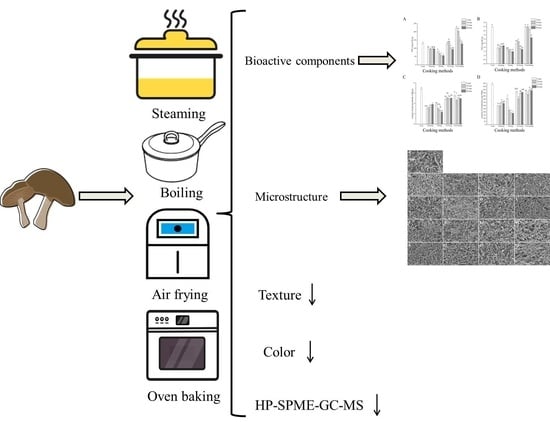
Effect of Different Cooking Methods on the Bioactive Components, Color, Texture, Microstructure, and Volatiles of Shiitake Mushrooms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Cooking Methods, and Sample Preparation
2.2. Analysis of Bioactive Components in Shiitake Mushrooms
2.2.1. Preparation and Determination of Total Phenol Content
2.2.2. Preparation and Determination of Total Flavonoid Content
2.2.3. Preparation and Determination Crude Polysaccharides Content
2.2.4. Preparation and HPLC Analysis of Eritadenine
2.3. Color Determination of Shiitake Mushroom Samples
2.4. Texture Analysis of Shiitake Mushroom Samples
2.5. SEM Observation of Shiitake Mushroom Samples
2.6. GC-MS Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Cooking Methods on Bioactive Component Contents
3.2. Color Properties of the Shiitake Mushroom
3.3. Texture
3.4. SEM Observation of Shiitake Mushrooms
3.5. HS-SPME-GC-MS Analysis of Volatile Compounds of Shiitake Mushrooms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muszyńska, B.; Kaa, K.; Wodarczyk, A.; Krakowska, A.; Suchoci, P. Lentinula edodes as a source of bioelements released into artificial digestive juices and potential anti-inflammatory material. Biol. Trace Elem. Res. 2020, 194, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Tiane, C.; Finimundy, T.; José, A.; Dillon, P.; Antônio, J.; Henriques, J.A.; Roesch Ely, M. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr. Sci. 2014, 5, 1095–1105. [Google Scholar]
- Sheng, K.; Wang, C.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Enman, J.; Rova, U.; Berglund, K.A. Quantification of the bioactive compound eritadenine in selected strains of shiitake mushroom (Lentinus edodes). J. Agr. Food Chem. 2007, 55, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Munyany, M.; Mduluza, T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 2016, 5, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, M.; Paula, V.B.; Olloqui, N.D.; García, D.F.; Combarros-Fuertes, P.; Estevinho, L.M.; Fresno-Baro, J.M. Effect of different cooking methods on the total phenolic content, antioxidant activity and sensory properties of wild Boletus edulis mushroom. Int. J. Gastron. Food Sci. 2021, 26, 100416. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agr. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Roncero-ramos, I.; Mendiola-lanao, M.; Pérez-clavijo, M.; Delgado-andrade, C. Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kajumba, P.K.; Okello, D.; Nyeinga, K.; Nydal, O.J. Assessment of the energy needs for cooking local food in Uganda: A strategy for sizing thermal energy storage with cooker system. Energy Sustain. Dev. 2022, 67, 67–80. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Varadharajan, S.; Siddhuraju, P. Different effects of processing methods on total phenolic content, antioxidant and antimicrobial activities of these species of Solanum. J. Food Drug Anal. 2012, 20, 844–854. [Google Scholar]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual. Food Science Texts Series; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; Chapter 6; pp. 47–53. [Google Scholar]
- Tanriseven, D.; Kadiroglu, P.; Selli, S.; Kelebek, H. LC-DAD-ESI-MS/MSassisted elucidation of the phenolic compounds in shalgams: Comparison of traditional and direct methods. Food Chem. 2020, 305, 125505. [Google Scholar] [CrossRef]
- Lespinard, A.R.; Goñi, S.M.; Salgado, P.R.; Mascheroni, R.H. Experimental determination and modelling of size variation, heat transfer and quality indexes during mushroom blanching. J. Food Eng. 2009, 92, 8–17. [Google Scholar] [CrossRef]
- Yin, C.; Fan, X.; Fan, Z. Comparison of non-volatile and volatile flavor compounds in six Pleurotus mushroom. J. Sci. Food Agr. 2019, 99, 1691–1699. [Google Scholar] [CrossRef]
- López-Hernández, A.A.; Ortega-Villarreal, A.S.; Vázquez-Rodríguez, J.A.; López-Cabanillas Lomelí, M.; González-Martínez, B.E. Application of different cooking methods to improve nutritional quality of broccoli (Brassica oleracea var. italica) regarding its compounds content with antioxidant activity. Int. J. Gastron. Food Sci. 2022, 28, 100510. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Wong, K.W.; Benzie, I.F. The effect of cooking on Brassica vegetables. Food Chem. 2008, 110, 706–710. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the key aroma and phenolic compounds of champignon (Agaricus bisporus) and oyster (Pleurotus ostreatus) mushrooms after two cooking treatments as elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef]
- Mashiane, P.; Mashitoa, F.M.; Slabbert, R.M.; Sivakumar, D. Impact of household cooking techniques on colour, antioxidant and sensory properties of African pumpkin and pumpkin leaves. Int. J. Gastron. Food Sci. 2021, 23, 100307. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agr. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.; Choi, Y.; Kim, Y.; Lee, J. Effect of Different Cooking Methods on the True Retention of Vitamins, Minerals, and Bioactive Compounds in Shiitake Mushrooms (Lentinula edodes). Korean J. Food Sci. Technol. Res. 2019, 25, 115–122. [Google Scholar] [CrossRef]
- Sori, I.; Yong-Gi, C.; Shaoting, L.; Jung-Ah, H. Antioxidative and nutritional characteristics of Shiitake mushrooms when cooked using different methods. Korean J. Food Sci. Technol. 2018, 50, 8–13. [Google Scholar]
- Sánchez-Minutti, L.; López-Valdez, F.; Rosales-Pérez, M.; Luna-Suárez, S. Effect of heat treatments of Lentinula edodes mushroom on eritadenine concentration. LWT-Food Sci. Technol. 2018, 18, 31112–311125. [Google Scholar] [CrossRef]
- Morales, D.; Tabernero, M.; Largo, C.; Polo, G.; Jiménez Piris, A.; Soler-Rivas, C. Effffect of traditional and modern culinary processing, bioaccessibility, biosafety and bioavailability of eritadenine, a hypocholesterolemic compound from edible mushrooms. Food Funct. 2018, 9, 6360. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef]
- Bojarska, U.; Batura, J.; Cierach, M. The effect of measurement site on the evaluation of tom breast muscle color. Pol. J. Food Nutr. Sci. 2003, 53, 45–49. [Google Scholar]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrovic, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- Nistor, O.V.; Stãnciuc, N.; Andronoiu, D.G.; Mocanu, G.D.; Botez, E. Ohmic treatment of apple puree (Golden delicious variety) in relation to product quality. Food Sci. Biotechnol. 2015, 24, 51–59. [Google Scholar] [CrossRef]
- Nketia, S.; Buckman, E.S.; Dzomeku, M.; Akonor, P.T. Effect of processing and storage on physical and texture qualities of oyster mushrooms canned in different media. Sci. Afr. 2020, 9, e00501. [Google Scholar] [CrossRef]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Bau, T.; Jin, R.X.; Cui, X.; Zhang, Y.T.; Kong, W.L. Comparison of different drying techniques for shiitake mushroom (Lentinus edodes): Change in volatile compounds, taste properties, and texture qualities. LWT-Food Sci. Technol. 2022, 164, 113651. [Google Scholar] [CrossRef]
- Paciulli, M.; Ganino, T.; Carini, E.; Pellegrini, N.; Pugliese, A.; Chiavaro, E. Effect of different cooking methods on structure and quality of industrially frozen carrots. J. Food Sci. Technol. 2016, 53, 2443–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, M.; Henríquez, C.; Escoar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynarascolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Lipan, L. Volatile composition and sensory profile of shiitake mushrooms as affected by drying method. J. Sci. Food Agr. 2018, 98, 1151–1521. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Li, R.; Yang, H.M.; Wang, S.Q.; Lin, R.; Tan, M.Q. Characterisation of moisture migration of shiitake mushroom (Lentinula edodes) during storage and its relationship to quality deterioration. Int. J. Food Sci. Technol. 2020, 55, 2132–2140. [Google Scholar] [CrossRef]
- Tian, Y.T.; Zhao, Y.T.; Huang, J.J.; Zeng, H.L.; Zheng, B.D. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]


| Cooking Methods | Cooking Time (min) | L* | a* | b* |
|---|---|---|---|---|
| Raw | 0 | 84.21 ± 2.92 a | 0.12 ± 0.08 ef | 6.34 ± 0.90 g |
| Steaming | 5 | 72.96 ± 4.62 de | 0.20 ± 0.03 ef | 12.3 ± 1.09 bc |
| 10 | 75.84 ± 1.82 bcd | 1.12 ± 0.13 abcd | 14.16 ± 0.94 a | |
| 15 | 77.63 ± 2.06 bcd | 1.24 ± 0.26 abc | 13.23 ± 1.31 ab | |
| 20 | 77.78 ± 3.61 bcde | 1.35 ± 0.34 ab | 12.95 ± 0.75 ab | |
| Boiling | 5 | 61.11 ± 8.05 e | 0.81 ± 0.02 g | 6.55 ± 1.65 g |
| 10 | 71.59 ± 2.25 de | 0.25 ± 0.03 ef | 9.86 ± 0.86 ef | |
| 15 | 64.97 ± 4.71 fe | 0.22 ± 0.08 ef | 10 ± 1.80 def | |
| 20 | 67.00 ± 4.35 fe | 1.35 ± 0.09 ab | 11.97 ± 2.23 bc | |
| Air frying | 5 | 72.05 ± 4.89 cde | 0.10 ± 0.03 f | 9.65 ± 1.89 f |
| 10 | 75.79 ± 3.18 bcd | 0.44 ± 0.03 def | 10.52 ± 0.85 cdef | |
| 15 | 75.38 ± 2.18 bc | 1.17 ± 0.10 abcd | 13.07 ± 0.48 ab | |
| 20 | 72.53 ± 4.19 bcde | 1.71 ± 0.05 a | 14.675 ± 0.67 a | |
| Oven baking | 5 | 71.24 ± 4.50 de | 0.93 ± 0.06 bcde | 11.77 ± 1.60 bcd |
| 10 | 75.23 ± 2.45 bcd | 0.53 ± 0.05 cdef | 11.60 ± 1.22 bcde | |
| 15 | 73.05 ± 3.69 bcde | 0.74 ± 0.06 bcdef | 12.19 ± 1.06 bc | |
| 20 | 72.63 ± 4.47 b | 0.80 ± 0.07 bcdef | 12.20 ± 1.63 bc |
| Cooking Methods | Cooking Time | Hardness (N) | Cohesivene | Gumminess (N) | Springiness (%) | Chewiness (N) | Adhesiveness (N.s) |
|---|---|---|---|---|---|---|---|
| Raw | 0 | 2007.33 ± 10.97 a | 0.76 ± 0.06 abc | 1516.20 ± 8.28 a | 0.88 ± 0.05 ab | 1328.70 ± 67.64 a | −1.23 ± 0.28 a |
| Steaming | 5 | 1594 ± 43.15 bc | 0.66 ± 0.03 fg | 1058.64 ± 28.66 cd | 0.73 ± 0.03 def | 826.90 ± 14.90 efg | −12.73 ± 0.73 cd |
| 10 | 1569.95 ± 219.01 bcd | 0.66 ± 0.03 fg | 1038.20 ± 144.83 cde | 0.77 ± 0.06 cdef | 808.72 ± 75.32 e | −13.41 ± 0.23 cd | |
| 15 | 1026.47 ± 116.70 fg | 0.67 ± 0.05 fg | 686.11 ± 78.00 fgh | 0.73 ± 0 cde | 538.84 ± 37.31 hi | −14.83 ± 0.46 cde | |
| 20 | 747.27 ± 24.05 g | 0.70 ± 0.03 def | 523.65 ± 16.85 h | 0.72 ± 0.02 abc | 423.16 ± 40.77 jk | −15.88 ± 1.50 def | |
| Boiling | 5 | 1748.98 ± 108.75 abc | 0.70 ± 0.05 cdef | 1228.29 ± 76.37 bc | 0.87 ± 0.03 ab | 1069.41 ± 37.83 c | −8.07 ± 1.58 abc |
| 10 | 1562.09 ± 151.00 bcd | 0.70 ± 0.05 def | 1092.11 ± 105.57 cd | 0.86 ± 0.04 ab | 933.86 ± 47.67 d | −11.36 ± 3.0 bcd | |
| 15 | 1235.42 ± 126.99 def | 0.71 ± 0.03 cdef | 876.30 ± 90.07 def | 0.84 ± 0.07 abc | 734.07 ± 63.60 fg | −21.79 ± 5.50 efg | |
| 20 | 1019.25 ± 226.12 fg | 0.67 ± 0.04 fg | 680.01 ± 150.86 fgh | 0.82 ± 0.06 bcd | 557.46 ± 38.49 h | −11.01 ± 2.54 bcd | |
| Air frying | 5 | 1210.65 ± 145.53 g | 0.77 ± 0.03 ab | 926.46 ± 111.37 def | 0.90 ± 0.03 a | 838.3062 ± 27.26 e | −9.85 ± 0.62 bcd |
| 10 | 1082.71 ± 135.43 efg | 0.73 ± 0.03 bcde | 792.98 ± 99.18 efg | 0.89 ± 0.05 a | 706.18 ± 37.00 g | −15.77 ± 2.70 def | |
| 15 | 789.47 ± 260.08 g | 0.75 ± 0.04 abcd | 590.95 ± 194.68 gh | 0.90 ± 0.04 a | 529.21 ± 23.52 hi | −23.11 ± 1.26 fg | |
| 20 | 751.44 ± 39.78 g | 0.62 ± 0.04 g | 467.58 ± 24.75 h | 0.80 ± 0.10 bcd | 374.53 ± 48.03 k | −26.01 ± 5.91 g | |
| Oven baking | 5 | 1803.00 ± 244.42 ab | 0.76 ± 0.03 a | 1442.90 ± 195.60 ab | 0.87 ± 0.06 ab | 1256.26 ± 89.33 b | −4.57 ± 1.71 ab |
| 10 | 1433.00 ± 81.00 cde | 0.76 ± 0.03 ab | 1095.53 ± 61.92 cd | 0.73 ± 0.08 def | 795.76 ± 92.76 ef | −16.06 ± 2.46 def | |
| 15 | 913.29 ± 117.54 fg | 0.74 ± 0.05 bcde | 681.45 ± 75.95 fgh | 0.70 ± 0.04 ef | 479.48 ± 25.75 ij | −26.17 ± 1.06 g | |
| 20 | 1012.62 ± 251.53 fg | 0.69 ± 0.06 ef | 702.16 ± 174.41 fgh | 0.70 ± 0.04 f | 489.51 ± 30.19 hij | −112.341 ± 16.08 h |
| Rentation Time (min) | Compounds | Relative Contents (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Steaming (min) | Boiling (min) | Air Frying (min) | Oven Baking (min) | |||||||||||||||
| 5 | 10 | 15 | 20 | 5 | 10 | 15 | 20 | 5 | 10 | 15 | 20 | 5 | 10 | 15 | 20 | ||||
| Alcohols | 27.38 | 3-Octanol | Nd | 0.60 | Nd | Nd | Nd | 0.12 | 0.32 | Nd | 0.27 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.67 |
| 29.78 | 1-Octen-3-ol | 41.32 | 6.36 | 2.26 | 1.68 | 1.01 | 4.50 | 21.88 | 15.32 | 0.67 | 2.24 | 3.65 | 4.39 | 1.20 | 0.65 | 0.25 | 0.28 | 0.32 | |
| 18.75 | Eucalyptol | 0.01 | 0.17 | 0.22 | 0.21 | Nd | Nd | 0.12 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 30.26 | 1-Heptanol | 0.13 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 31.49 | 1-Hexanol, 2-ethyl- | 0.04 | 0.36 | 0.55 | 0.51 | 0.41 | Nd | 0.29 | 0.79 | 0.27 | Nd | Nd | Nd | Nd | 0.43 | Nd | 0.31 | Nd | |
| 34.31 | 1-Octanol | 2.54 | Nd | 0.38 | 0.27 | Nd | Nd | 0.26 | Nd | Nd | 0.37 | 0.35 | Nd | 0.12 | Nd | Nd | 0.15 | Nd | |
| 35.41 | 3-Octen-1-ol | 0.01 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 36.51 | trans-2-Undecen-1-ol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.11 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 36.52 | Cyclooctyl alcohol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.20 | Nd | Nd | Nd | Nd | Nd | |
| 36.62 | 2-Octen-1-ol, (E)- | 0.80 | Nd | Nd | Nd | Nd | 0.07 | 0.18 | Nd | Nd | Nd | Nd | Nd | Nd | 0.13 | Nd | Nd | Nd | |
| 36.63 | trans-2-Undecen-1-ol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 36.66 | 2-Nonen-1-ol, (E)- | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 4.36 | Nd | Nd | Nd | Nd | Nd | |
| 38.35 | 1-Nonanol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.27 | 0.22 | Nd | Nd | Nd | Nd | Nd | Nd | |
| 47.66 | Phenylethyl Alcohol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.05 | Nd | 0.07 | Nd | Nd | Nd | Nd | Nd | |
| 54.31 | Cedrol | Nd | 0.02 | 0.27 | 0.23 | 0.27 | Nd | Nd | Nd | 0.15 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Total | 44.891 | 7.50 | 3.68 | 2.90 | 1.70 | 4.68 | 23.06 | 16.11 | 1.36 | 3.04 | 4.22 | 9.02 | 2.12 | 1.35 | 0.39 | 0.74 | 0.99 | ||
| Aldehydes | 37.95 | Benzeneacetaldehyde | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.25 | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| 13.44 | Hexanal | Nd | 1.57 | 4.69 | 8.63 | 3.19 | 0.22 | 1.19 | 3.44 | 1.74 | Nd | Nd | 0.40 | 0.40 | 1.30 | 0.90 | 0.90 | 1.01 | |
| 17.62 | Heptanal | Nd | 0.35 | 0.52 | 0.51 | 0.40 | 0.37 | 0.55 | 0.22 | 0.08 | 0.07 | 0.09 | Nd | 0.11 | Nd | 0.21 | 0.12 | ||
| 22.36 | Octanal | 0.23 | 0.80 | 0.76 | 0.67 | 0.71 | 0.06 | 0.29 | 0.41 | 0.16 | 0.36 | 0.29 | 0.20 | 0.08 | 0.28 | 0.29 | 0.32 | 0.21 | |
| 24.28 | 2-Heptenal, (E)- | Nd | 0.24 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.14 | Nd | Nd | |
| 27.11 | Nonanal | Nd | 0.24 | 2.40 | 1.79 | 2.61 | 2.39 | 1.21 | 1.70 | 0.90 | 2.31 | 1.70 | 1.37 | Nd | 1.63 | Nd | 1.61 | 0.94 | |
| 28.83 | 2-Octenal, (E)- | 2.40 | 0.24 | 0.12 | 0.20 | Nd | Nd | 0.12 | Nd | Nd | 0.95 | Nd | 1.88 | 0.44 | Nd | 0.12 | Nd | Nd | |
| 28.87 | 2-Dodecenal | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.32 | Nd | 0.17 | Nd | |
| 31.68 | Decanal | 0.13 | 0.49 | 0.37 | 0.52 | 0.33 | 0.17 | 0.28 | Nd | 0.42 | 0.31 | 0.21 | 0.25 | 0.15 | 0.45 | 0.30 | Nd | 0.40 | |
| 32.92 | Benzaldehyde | 0.04 | 0.21 | 0.34 | Nd | Nd | Nd | 0.13 | Nd | Nd | 0.23 | 0.12 | 0.38 | 0.10 | Nd | Nd | 0.19 | 0.10 | |
| 33.34 | 2-Nonenal, (E)- | Nd | 0.32 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.18 | 0.33 | Nd | Nd | 0.31 | Nd | 0.22 | 0.16 | |
| 35.64 | 2,4-Octadienal, (E,E)- | 0.03 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 43.70 | 2-Phenylpropenal | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.07 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Total | 2.82 | 4.46 | 9.20 | 12.33 | 7.25 | 2.84 | 3.60 | 6.10 | 3.44 | 4.50 | 2.72 | 4.57 | 1.18 | 4.39 | 1.75 | 3.63 | 2.93 | ||
| Ketones | 20.86 | 3-Octanone | 8.83 | 1.47 | 0.76 | 2.84 | Nd | Nd | 2.22 | 1.26 | 0.18 | Nd | 7.73 | 8.96 | 1.25 | 0.36 | 0.14 | 0.23 | 0.99 |
| 23.11 | 1-Octen-3-one | 6.81 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 1.38 | Nd | 3.08 | 1.10 | Nd | Nd | Nd | Nd | |
| 24.76 | 5-Hepten-2-one, 6-methyl- | Nd | 0.12 | Nd | Nd | Nd | Nd | Nd | Nd | 0.28 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 31.57 | 2-Decanone | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.40 | 0.29 | |
| 35.86 | 2-Undecanone | 0.04 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.07 | 0.08 | 0.06 | Nd | Nd | Nd | Nd | Nd | |
| 38.08 | Acetophenone | 0.05 | 0.27 | 0.47 | 1.74 | 0.34 | Nd | 0.16 | 0.47 | Nd | Nd | 0.13 | 0.18 | Nd | 0.19 | Nd | Nd | Nd | |
| Total | 15.74 | 1.86 | 1.22 | 4.58 | 0.34 | Nd | 2.38 | 1.73 | 0.47 | 1.4424 | 7.94 | 12.29 | 2.35 | 0.55 | 0.14 | 0.63 | 1.29 | ||
| Sulfur | 13.12 | Disulfide, dimethyl | 1.69 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 4.74 | 1.75 | 0.88 | 0.42 | Nd | Nd | Nd | Nd |
| 27.00 | Thiourea | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.10 | 0.03 | Nd | Nd | Nd | Nd | Nd | Nd | |
| 27.14 | Carbonochloridodithioic acid, methyl ester | 0.23 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 38.54 | Disulfide, methyl (methylthio)methyl | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.18 | Nd | Nd | Nd | Nd | Nd | |
| 26.74 | Dimethyl trisulfide | 3.51 | 0.10 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 27.72 | 15.44 | 7.63 | 1.27 | Nd | Nd | Nd | Nd | |
| Total | 5.43 | 0.10 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 32.56 | 17.22 | 8.70 | 1.69 | Nd | Nd | Nd | Nd | ||
| Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |||
| Benzene | 11.90 | Toluene | Nd | Nd | Nd | Nd | Nd | 0.12 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| 41.84 | Oxime-, methoxy-phenyl-_ | 2.14 | 10.53 | 11.96 | 14.42 | 14.51 | 8.03 | 20.34 | 13.16 | 21.02 | 6.89 | 7.67 | 6.35 | 8.60 | 12.55 | 26.42 | 20.0 | 17.79 | |
| 53.54 | Butylated Hydroxytoluene | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.08 | Nd | Nd | Nd | |
| Total | 2.14 | 10.53 | 11.96 | 14.42 | 14.51 | 8.15 | 20.34 | 13.16 | 21.02 | 6.89 | 7.67 | 6.35 | 8.60 | 12.63 | 26.42 | 20.70 | 17.79 | ||
| Alkanes | 25.07 | Dodecane, 2,6,10-trimethyl- | Nd | Nd | Nd | Nd | Nd | 0.06 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| 27.08 | Tetradecane | Nd | Nd | Nd | Nd | Nd | 0.10 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 1.23 | Nd | Nd | |
| 31.39 | Pentadecane | 0.03 | Nd | Nd | Nd | Nd | Nd | 0.14 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 31.66 | Pentadecane | 0.04 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 35.63 | Hexadecane | 0.06 | Nd | 0.21 | Nd | Nd | Nd | Nd | Nd | Nd | 0.06 | 0.07 | Nd | Nd | 0.10 | Nd | Nd | Nd | |
| 39.67 | Heptadecane | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 40.25 | Octadecanal | 0.04 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 47.88 | Octadecanal | 0.05 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 49.41 | Cyclododecane | 0.23 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Total | Nd | Nd | 0.21 | Nd | Nd | 0.17 | 0.14 | Nd | Nd | 0.06 | 0.07 | Nd | Nd | 0.10 | 1.23 | Nd | Nd | ||
| Alkenes | 9.19 | 1,3-Octadiene | 0.03 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd |
| 9.27 | Cyclooctene | 0.03 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 17.87 | D-Limonene | Nd | Nd | Nd | Nd | Nd | 0.12 | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 38.35 | 1-Decene | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.18 | Nd | Nd | Nd | Nd | Nd | |
| Total | 0.06 | Nd | Nd | Nd | Nd | 0.12 | Nd | Nd | Nd | Nd | Nd | 0.18 | Nd | Nd | Nd | Nd | Nd | ||
| Others | 19.58 | Furan, 2-pentyl- | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.41 | 0.17 | Nd | Nd | Nd | Nd | 0.07 | Nd | Nd | Nd |
| 34.30 | Formic acid, octyl ester | Nd | 0.30 | Nd | Nd | 0.36 | 0.10 | Nd | Nd | Nd | Nd | Nd | 0.25 | Nd | 0.14 | Nd | Nd | Nd | |
| 38.37 | Nonyl chloroformate | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| 50.80 | Phenol | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | 0.17 | |
| 57.47 | Phenol, 2,6-bis(1,1-dimethylethyl)- | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | Nd | |
| Total | Nd | 0.46 | 0.40 | Nd | Nd | 0.18 | Nd | Nd | Nd | 0.09 | Nd | Nd | Nd | Nd | Nd | Nd | 0.09 | ||
| Total | 71.60 | 24.76 | 26.73 | 34.64 | 24.16 | 8.04 | 49.68 | 37.52 | 26.46 | 48.49 | 39.93 | 41.35 | 15.93 | 19.23 | 29.94 | 25.42 | 22.99 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, F.; Gao, H.; Yin, C.-M.; Shi, D.-F.; Fan, X.-Z. Effect of Different Cooking Methods on the Bioactive Components, Color, Texture, Microstructure, and Volatiles of Shiitake Mushrooms. Foods 2023, 12, 2573. https://doi.org/10.3390/foods12132573
Yao F, Gao H, Yin C-M, Shi D-F, Fan X-Z. Effect of Different Cooking Methods on the Bioactive Components, Color, Texture, Microstructure, and Volatiles of Shiitake Mushrooms. Foods. 2023; 12(13):2573. https://doi.org/10.3390/foods12132573
Chicago/Turabian StyleYao, Fen, Hong Gao, Chao-Min Yin, De-Fang Shi, and Xiu-Zhi Fan. 2023. "Effect of Different Cooking Methods on the Bioactive Components, Color, Texture, Microstructure, and Volatiles of Shiitake Mushrooms" Foods 12, no. 13: 2573. https://doi.org/10.3390/foods12132573