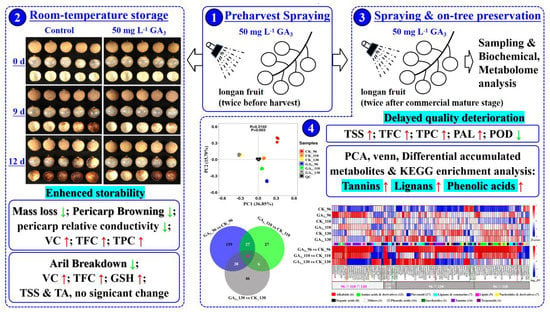
GA3 Treatment Delays the Deterioration of ‘Shixia’ Longan during the On-Tree Preservation and Room-Temperature Storage and Up-Regulates Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preharvest Spraying Treatments and Fruit Harvest
2.2. Determination of Total Soluble Solids (TSS) Content, TFC, and TPC
2.3. Measurement of the Activity of Phenylalanine Ammonia-Lyase (PAL) and Peroxidase (POD)
2.4. Widely Targeted Metabolome Analysis of On-Tree Preserved Longan Pulp Samples
2.5. Postharvest Treatment, Storage, and Sampling
2.6. Determination of Mass Loss, Pericarp Relative Conductivity, Pericarp Browning Index, and Aril Breakdown Index
2.7. Determination of TSS, TA, Vitamin C, and Reduced Glutathione (GSH) in Postharvest Longan Samples
2.8. Statistical Analysis
3. Results and Discussion
3.1. GA3 Treatment Affects TSS, Antioxidants, and Related Enzyme Activities in Longan Fruit during Ripening and On-Tree Preservation
3.2. Identification, Quantification, and Classification of Metabolites Detected in the GA3-Treated and Control Longan Pulp
3.3. Significantly Differently Accumulated Metabolites between the GA3-Treated and Control Longan Pulp
3.4. KEGG Enrichment of DAMs and Display of Important Metabolic Pathways
3.5. The Effect of Preharvest 50 mg L−1 GA3 Treatment on Storability and Postharvest Fruits’ Antioxidants during Room-Temperature Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, T.; Niu, J.J.; Guo, X.M.; Wu, H.T.; Han, D.M.; Shuai, L.; Wu, Z.X. Preharvest Zinc Sulfate Spray Improves the Storability of Longan (Dimocarpus longan Lour.) Fruits by Protecting the Cell Wall Components and Antioxidants of Pericarp: Preharvest ZnSO4 Spray Improves the Storage Performance of Longan Fruits. J. Sci. Food Agric. 2019, 99, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Shuai, L.; Han, D.M.; Lai, Z.Y.; Du, X.X.; Guo, X.M.; Hu, W.S.; Wu, Z.X.; Luo, T. Comparative Metabolomics Reveals Differences in Primary and Secondary Metabolites between “Shixia” and “Chuliang” Longan (Dimocarpus longan Lour.) Pulp. Food Sci. Nutr. 2021, 9, 5785–5799. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Shuai, L.; Liao, L.Y.; Li, J.; Duan, Z.H.; Guo, X.M.; Xue, X.Q.; Han, D.M.; Wu, Z.X. Soluble Acid Invertases Act as Key Factors Influencing the Sucrose/Hexose Ratio and Sugar Receding in Longan (Dimocarpus longan Lour.) Pulp. J. Agric. Food Chem. 2019, 67, 352–363. [Google Scholar] [CrossRef]
- Lin, Y.L.; Min, J.M.; Lai, R.L.; Wu, Z.Y.; Chen, Y.K.; Yu, L.L.; Cheng, C.Z.; Jin, Y.C.; Tian, Q.L.; Liu, Q.F.; et al. Genome-Wide Sequencing of Longan (Dimocarpus longan Lour.) Provides Insights into Molecular Basis of Its Polyphenol-Rich Characteristics. GigaScience 2017, 6, gix023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Guo, S.; Ho, C.-T.; Bai, N.S. Phytochemical Constituents and Biological Activities of Longan (Dimocarpus longan Lour.) Fruit: A Review. Food Sci. Hum. Wellness 2020, 9, 95–102. [Google Scholar] [CrossRef]
- Mao, Y.Z.; Yang, H.S.; Fu, C.L.; You, S.Y.; Cao, H.; Lai, S.J. Recent Advances in Longan Polysaccharides and Polyphenols. In Asian Berries: Health Benefits, 1st ed.; Xiao, G.S., Xu, Y.J., Yu, Y.S., Eds.; CRC Press: Boca Raton, FL, USA, 2020; Chapter 8; pp. 149–172. ISBN 978-0-429-28647-6. [Google Scholar] [CrossRef]
- Luo, T.; Yin, F.L.; Liao, L.Y.; Liu, Y.F.; Guan, B.Y.; Wang, M.; Lai, T.T.; Wu, Z.X.; Shuai, L. Postharvest Melatonin Treatment Inhibited Longan (Dimocarpus longan Lour.) Pericarp Browning by Increasing ROS Scavenging Ability and Protecting Cytomembrane Integrity. Food Sci. Nutr. 2021, 9, 4963–4973. [Google Scholar] [CrossRef]
- Han, D.M.; Luo, T.; Zhang, L.; Wu, J.Q.; Wu, H.T.; Wu, Z.X.; Li, J.G.; Wang, J.; Pan, X.W. Optimized Precooling Combined with SO2-released Paper Treatment Improves the Storability of Longan (Dimocarpus longan Lour.) Fruits Stored at Room Temperature. Food Sci. Nutr. 2020, 8, 2827–2838. [Google Scholar] [CrossRef]
- Xiang, W.J.; Wang, H.-W.; Sun, D.-W. Phytohormones in Postharvest Storage of Fruit and Vegetables: Mechanisms and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 2969–2983. [Google Scholar] [CrossRef]
- Kapłan, M.; Najda, A.B.; Klimek, K.; Borowy, A. Effect of Gibberellic Acid (GA3) Inflorescence Application on Content of Bioactive Compounds and Antioxidant Potential of Grape (Vitis L.) ‘Einset Seedless’ Berries. S. Afr. J. Enol. Vitic. 2019, 40, 1–10. [Google Scholar] [CrossRef]
- Montero, T.; Mollá, E.; Martín-Cabrejas, M.A.; López-Andréu, F.J. Effects of Gibberellic Acid (GA3) on Strawberry PAL (Phenylalanine Ammonia-Lyase) and TAL (Tyrosine Ammonia-Lyase) Enzyme Activities. J. Sci. Food Agric. 1998, 77, 230–234. [Google Scholar] [CrossRef]
- Southwick, S.M.; Moran, R.E.; Yeager, J.T.; Glozer, K. Use of Gibberellin to Delay Maturity and Improve Fruit Quality of ‘French‘ Prune. J. Hortic. Sci. Biotechnol. 2000, 75, 591–597. [Google Scholar] [CrossRef]
- Tian, S.F.; Wang, Y.; Du, G.; Li, Y.X. Changes in Contents and Antioxidant Activity of Phenolic Compounds during Gibberellin-Induced Development in Vitis vinifera L. ‘Muscat’. Acta Physiol. Plant. 2011, 33, 2467–2475. [Google Scholar] [CrossRef]
- Yang, S.-L.; Zhang, X.-N.; Lu, G.-L.; Wang, C.-R.; Wang, R. Regulation of Gibberellin on Gene Expressions Related with the Lignin Biosynthesis in ‘Wangkumbae’ Pear (Pyrus pyrifolia Nakai) Fruit. Plant Growth Regul. 2014, 76, 127–134. [Google Scholar] [CrossRef]
- Huang, H.; Jing, G.X.; Wang, H.; Duan, X.W.; Qu, H.X.; Jiang, Y.M. The Combined Effects of Phenylurea and Gibberellins on Quality Maintenance and Shelf Life Extension of Banana Fruit during Storage. Sci. Hortic. 2014, 167, 36–42. [Google Scholar] [CrossRef]
- Canli, F.A.; Sahin, M.; Ercisli, S.; Yilmaz, O.; Temurtas, N.; Pektas, M. Harvest and Postharvest Quality of Sweet Cherry Are Improved by Pre-Harvest Benzyladenine and Benzyladenine plus Gibberellin Applications. J. Appl. Bot. Food Qual. 2015, 88, 255–258. [Google Scholar]
- Ozkan, Y.; Ucar, M.; Yildiz, K.; Ozturk, B. Pre-harvest Gibberellic Acid (GA3) Treatments Play an Important Role on Bioactive Compounds and Fruit Quality of Sweet Cherry Cultivars. Sci. Hortic. 2016, 211, 358–362. [Google Scholar] [CrossRef]
- Kurlus, R.; Świerczyński, S.; Rutkowski, K.; Ratajkiewicz, H.; Malinowska, A.; Wyrwał, A. Exogenus ‘GA3’ and ‘GA4+7’ effects on phenological indices, frost hardiness and quality properties of ‘English morello’ sour cherry (Prunus cerasus L.). Acta Sci. Pol. Hortorum Cultus 2017, 16, 99–109. [Google Scholar] [CrossRef]
- Elmenofy, H.M.; Kheder, A.; Mansour, A.H.A. Improvement of Fruit Quality and Marketability of “Washington Navel” Orange Fruit by Cytokinin and Gibberellin. Egypt. J. Hortic. 2021, 48, 141–156. [Google Scholar] [CrossRef]
- Ozturk, B.; Bektas, E.; Aglar, E.; Karakaya, O.; Gun, S.F. Cracking and Quality Attributes of Jujube Fruits as Affected by Covering and Pre-Harvest Parka and GA3 Treatments. Sci. Hortic. 2018, 240, 65–71. [Google Scholar] [CrossRef]
- Liu, L.Q.; Li, J.G.; Shu, B.; Wang, Y.C.; Wu, H.X.; Jue, D.W.; Shi, S.Y. Effects of GA3 on Sugar Accumulation and Sugar Metabolism-Related Enzyme Activities in Shixia of Dimocarpus longan Lour Fruits. Southwest China J. Agric. Sci. 2016, 29, 2698–2703. (In Chinese) [Google Scholar] [CrossRef]
- Tan, S.; Xie, J.H.; Wang, W.; Shi, S.Y. Effects of Exogenous Plant Hormones on Sugar Accumulation and Related Enzyme Activities during the Development of Longan (Dimocarpus longan Lour.) Fruits. J. Hortic. Sci. Biotechnol. 2019, 94, 790–797. [Google Scholar] [CrossRef]
- Shuai, L.; Liu, H.; Liao, L.Y.; Lai, T.T.; Lai, Z.Y.; Du, X.X.; Duan, Z.H.; Wu, Z.X.; Luo, T. Widely Targeted Metabolic Analysis Revealed the Changed Pigmentation and Bioactive Compounds in the Ripening Berchemia floribunda (Wall.) Brongn. Fruit. Food Sci. Nutr. 2021, 9, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, B.K. An Important Role of the Pepper Phenylalanine Ammonia-Lyase Gene (PAL1) in Salicylic Acid-Dependent Signalling of the Defence Response to Microbial Pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Lin, H.T.; Lin, Y.X.; Zhang, S.; Chen, Y.H.; Jiang, X.J. The Roles of Metabolism of Membrane Lipids and Phenolics in Hydrogen Peroxide-Induced Pericarp Browning of Harvested Longan Fruit. Postharvest Biol. Technol. 2016, 111, 53–61. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography−Mass Spectrometry, XCMS, and Chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Eriksson, L.; Andersson, P.L.; Johansson, E.; Tysklind, M. Megavariate Analysis of Environmental QSAR Data. Part I—A Basic Framework Founded on Principal Component Analysis (PCA), Partial Least Squares (PLS), and Statistical Molecular Design (SMD). Mol. Divers. 2006, 10, 169–186. [Google Scholar] [CrossRef]
- López-Ibáñez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0—Functional Enrichment of Chemical Compounds. Nucleic Acids Res. 2016, 44, W201–W204. [Google Scholar] [CrossRef]
- Lin, Y.F.; Lin, H.T.; Zhang, S.; Chen, Y.H.; Chen, M.Y.; Lin, Y.X. The Role of Active Oxygen Metabolism in Hydrogen Peroxide-Induced Pericarp Browning of Harvested Longan Fruit. Postharvest Biol. Technol. 2014, 96, 42–48. [Google Scholar] [CrossRef]
- Wang, J.; Guo, D.L.; Han, D.M.; Pan, X.W.; Li, J.G. A Comprehensive Insight into the Metabolic Landscape of Fruit Pulp, Peel, and Seed in Two Longan (Dimocarpus longan Lour.) Varieties. Int. J. Food Prop. 2020, 23, 1527–1539. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest Application of Oxalic Acid Increased Fruit Size, Bioactive Compounds, and Antioxidant Capacity in Sweet Cherry Cultivars (Prunus avium L.). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef]
- Serna-Escolano, V.; Giménez, M.J.; Castillo, S.; Valverde, J.M.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D.; Zapata, P.J. Preharvest Treatment with Oxalic Acid Improves Postharvest Storage of Lemon Fruit by Stimulation of the Antioxidant System and Phenolic Content. Antioxidants 2021, 10, 963. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valverde, J.M.; Valero, D.; Serrano, M. Melatonin Treatment to Pomegranate Trees Enhances Fruit Bioactive Compounds and Quality Traits at Harvest and during Postharvest Storage. Antioxidants 2021, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Zapata, P.J.; Martínez-Esplá, A.; Guillén, F.; Díaz-Mula, H.M.; Martínez-Romero, D.; Serrano, M.; Valero, D. Preharvest Application of Methyl Jasmonate (MeJA) in Two Plum Cultivars. 2. Improvement of Fruit Quality and Antioxidant Systems during Postharvest Storage. Postharvest Biol. Technol. 2014, 98, 115–122. [Google Scholar] [CrossRef]
- Duan, X.W.; Su, X.G.; You, Y.L.; Qu, H.X.; Li, Y.B.; Jiang, Y.M. Effect of Nitric Oxide on Pericarp Browning of Harvested Longan Fruit in Relation to Phenolic Metabolism. Food Chem. 2007, 104, 571–576. [Google Scholar] [CrossRef]
- Luo, T.; Xu, K.Y.; Luo, Y.; Chen, J.J.; Sheng, L.; Wang, J.Q.; Han, J.W.; Zeng, Y.L.; Xu, J.; Chen, J.M.; et al. Distinct Carotenoid and Flavonoid Accumulation in a Spontaneous Mutant of Ponkan (Citrus reticulata Blanco) Results in Yellowish Fruit and Enhanced Postharvest Resistance. J. Agric. Food Chem. 2015, 63, 8601–8614. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Lin, H.T.; Fan, Z.Q.; Wang, H.; Lin, M.S.; Chen, Y.H.; Hung, Y.-C.; Lin, Y.F. Inhibitory Effect of Propyl Gallate on Pulp Breakdown of Longan Fruit and Its Relationship with ROS Metabolism. Postharvest Biol. Technol. 2020, 168, 111272. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Lin, X.; Lai, T.; Long, L.; Lai, Z.; Du, X.; Guo, X.; Shuai, L.; Han, D.; Wu, Z. GA3 Treatment Delays the Deterioration of ‘Shixia’ Longan during the On-Tree Preservation and Room-Temperature Storage and Up-Regulates Antioxidants. Foods 2023, 12, 2032. https://doi.org/10.3390/foods12102032
Luo T, Lin X, Lai T, Long L, Lai Z, Du X, Guo X, Shuai L, Han D, Wu Z. GA3 Treatment Delays the Deterioration of ‘Shixia’ Longan during the On-Tree Preservation and Room-Temperature Storage and Up-Regulates Antioxidants. Foods. 2023; 12(10):2032. https://doi.org/10.3390/foods12102032
Chicago/Turabian StyleLuo, Tao, Xiaolan Lin, Tingting Lai, Libing Long, Ziying Lai, Xinxin Du, Xiaomeng Guo, Liang Shuai, Dongmei Han, and Zhenxian Wu. 2023. "GA3 Treatment Delays the Deterioration of ‘Shixia’ Longan during the On-Tree Preservation and Room-Temperature Storage and Up-Regulates Antioxidants" Foods 12, no. 10: 2032. https://doi.org/10.3390/foods12102032