Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference
Abstract
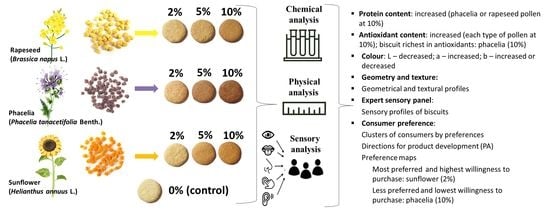
:1. Introduction
2. Materials and Methods
2.1. Raw Materials of Biscuits
2.2. Pollen Identification
2.3. Biscuit Preparation
2.4. Determination of Macronutrients
2.5. Determination of the Total Phenolic Content and In Vitro Antioxidant Capacity
2.5.1. Extract Preparation
2.5.2. Total Phenolic Content
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.5.5. 2,2-Diphenyl-1-picryhydrazyl (DPPH) Assay
2.6. Spectral Colour Measurement
2.7. Baking Loss, Geometry
2.8. Texture Analysis
2.9. Sensory Tests
2.9.1. Sensory Profile Analysis
2.9.2. Consumers’ Preference Tests
2.10. Statistical Analysis
3. Results and Discussion
3.1. Botanical Origin of Pollen Samples
3.2. Nutritional Properties of Biscuits
3.2.1. Macronutrient Composition
3.2.2. Total Phenolic Content and Antioxidant Properties
3.3. CIELAB Colour Parameters
3.4. Geometric Properties, Baking Loss
3.5. Texture Parameters
3.6. Sensory Attributes
3.6.1. Sensory Profile of Biscuits
3.6.2. Consumers’ Liking of Biscuits
3.6.3. Penalty Analysis
3.6.4. External Preference Map
3.6.5. Consumers’ Purchase Intention
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camacho-Bernal, G.I.; Cruz-Cansino, N.S.; Remírez-Moreno, E.; Delgado-Olivares, L.; Zafra-Rojas, Q.Y.; Castaneda-Ovando, A.; Suárez-Jacobo, Á. Addition of bee products in diverse food sources: Functional and physochemical properties. Appl. Sci. 2021, 11, 8156. [Google Scholar] [CrossRef]
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Oliveira, J.J.; Ribeiro, H. Food market trends: The cases of spirulina and bee pollen. In Proceedings of the 56th International Scientific Conference on Economic and Social Development, Aveiro, Portugal, 2–3 July 2020; pp. 246–258. [Google Scholar]
- Zuluaga, C.; Martínez, A.; Fernández, J.; López-Baldó, J.; Quiles, A.; Rodrigo, D. Effect of high pressure processing on carotenoid and phenolic compounds, antioxidant capacity, and microbial counts of bee-pollen paste and bee-pollen-based beverage. Innov. Food Sci. Emerg. Technol. 2016, 37, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Shariati, M.A.; Tesić, Ž.L.; Pesić, M.B. The application of pollen as a functional food and feed ingredient—The present and perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Sipos, L.; Végh, R.; Bodor, Z.; Zaukuu, J.-L.Z.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of bee pollen and prediction of sensory and colorimetric attributes—A sensometric fusion approach by e-nose, e-tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef]
- Prđun, S.; Svečnjak, L.; Valentić, M.; Marijanović, Z.; Jerković, I. Characterization of Bee Pollen: Physico-Chemical Properties, Headspace Composition and FTIR Spectral Profiles. Foods 2021, 10, 2103. [Google Scholar] [CrossRef]
- Campos, M.; Bogdanov, S.; Almedia-Muradian, L.B.; Szesna, T.; Mancebo, Y.; Frigerio, C.; Ferriera, F. Pollen composition and standardisation of analytical methods. Int. Bee Res. Assoc. 2008, 47, 154–161. [Google Scholar]
- Leinonen, I.; Iannetta, P.P.M.; Rees, R.M.; Russel, W.; Watson, C.; Barnes, A.P. Lysine supply is a critical factor in achieving sustainable global protein economy. Front. Sustain. Food Syst. 2019, 3, 27. [Google Scholar] [CrossRef]
- Dong, J.; Yang, Y.; Wang, X.; Zhang, H. Fatty acid profiles of 20 species of monofloral bee pollen from China. J. Apic. Res. 2016, 54, 503–511. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Pešić, M.B.; Mosić, M.D.; Dojčinović, B.P.; Natić, M.M.; Trifković, J.Đ. Mineral content of bee pollen from Serbia. Arh. Za Hig. Rada Toksikol. 2015, 66, 251–258. [Google Scholar] [CrossRef]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernández, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee products: An emblemetic example of underutilized sources of bioactive compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of Romanian Bee Pollen—An Important Nutritional Source. Foods 2022, 11, 2633. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic properties of bioactive compounds from different honeybee products. Front. Pharmacol. 2017, 28, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostić, A.Ž.; Barać, M.B.; Stanojević, S.P.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Šikoparija, B.; Radišić, P.; Prentović, M.; Pešić, M. Physicochemical composition and techno-functional properties of bee pollen collected in Serbia. LWT—Food Sci. Technol. 2015, 62, 301–309. [Google Scholar] [CrossRef]
- Arepally, D.; Reddy, R.S.; Goswami, T.K.; Datta, A.K. Biscuit baking: A review. LWT—Food Sci. Technol. 2020, 131, 109726. [Google Scholar] [CrossRef]
- Conte, P.; Caro, A.; Balestra, F.; Piga, A.; Fadda, C. Bee Pollen as a functional ingredient in gluten-free bread: A physical-chemical, technological and sensory approach. LWT—Food Sci. Technol. 2018, 90, 1–7. [Google Scholar] [CrossRef]
- Dundar, A.N. Total phenolic and antioxidant bioaccessibilities of cookies enriched with bee pollen. J. Food Process. Preserv. 2021, 46, e16085. [Google Scholar] [CrossRef]
- Krystyjan, M.; Gumul, D.; Ziobro, R.; Korus, A. The fortification of biscuits with bee pollen and its effect on physicochemical and antioxidant properties in biscuits. LWT—Food Sci. Technol. 2015, 63, 640–646. [Google Scholar] [CrossRef]
- Solgajová, M.; Nôžková, J.; Kadáková, M. Quality of durable cookies enriched with rape bee pollen. J. Central Eur. Agric. 2014, 15, 24–38. [Google Scholar] [CrossRef]
- von der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- AACC. Approved Method 10-50D: Baking Quality of Cookie Flour; AACC: Washington, DC, USA, 1980. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colometry of total phenolics with phosphomolibdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, M.S. Antioxidant determination by the use of stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root-their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [Green Version]
- CIE (Commission Internationale de l’Éclairage). Technical Report: Colorimetry, 3rd ed.; CIE: Washington, DC, USA, 2004. [Google Scholar]
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO (International Organization for Standardization): Geneva, Switzerland, 2007.
- ISO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO (International Organization for Standardization): Geneva, Switzerland, 2012.
- ISO 11132:2012; Sensory Analysis—Methodology—Guidelines for Monitoring the Performance of a Quantitative Sensory Panel. ISO (International Organization for Standardization): Geneva, Switzerland, 2012.
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO (International Organization for Standardization): Geneva, Switzerland, 2016.
- ISO 11136:2014/AMD 1:2020; Sensory Analysis–Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area—Amendment 1. ISO (International Organization for Standardization): Geneva, Switzerland, 2014.
- ISO 11136:2014; Sensory Analysis–Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. ISO (International Organization for Standardization): Geneva, Switzerland, 2014.
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 276–277; 407–412. [Google Scholar]
- Peltier, C.; Brockhoff, P.B.; Visalli, M.; Schlich, P. The MAM-CAP table: A new tool for monitoring panel performances. Food Qual. Pref. 2013, 32, 24–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 19 October 2022).
- McEwan, J.A. Preference mapping for product optimization. Data Handl. Sci. Technol. 1996, 16, 71–102. [Google Scholar]
- Végh, R.; Puter, D.; Vaskó, Á.; Csóka, M.; Mednyánszky, Z. Examination of the nutrient content and color characteristics of honey and pollen samples. J. Food Investig. 2022, 68, 3793–3806. [Google Scholar]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P.L. Characterization of Chemical Composition of Bee Pollen in China. J. Food Agric. Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of bee pollen extraction to maximise extractable antioxidant constituents. Antioxidants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gao, K.; Wang, K.; Xu, X.; Zhang, H. Cell wall disruption of rape bee pollen treated with combination of protamex hydrolysis and ultrasonication. Food Res. Int. 2015, 75, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Matamoro-Vidal, A.; Raquin, C.; Brisset, F.; Colas, H.; Izac, B.; Albert, B.; Gouyon, P.H. Links between morphology and function of the pollen wall: An experimental approach. Bot. J. Linn. Soc. 2016, 180, 478–490. [Google Scholar] [CrossRef] [Green Version]
- Jakobek, L. Interactions of polyphenols with carbohydrates, proteins and lipids. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Stanisavljević, N.S.; Gašić, U.M.; Lević, S.; Kojić, M.O.; Tešić, Ž.L.; Nedović, V.; Barać, M.B.; Pesić, M.B. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef]
- Starowitz, M.; Zeliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Bleha, R.; Shevtsova, T.V.; Živčáková, M.; Korbářová, A.; Ježková, M.; Saloň, I.; Brindza, J.; Synytsya, A. Spectroscopic Discrimination of Bee Pollen by Composition, Color, and Botanical Origin. Foods 2021, 10, 1682. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference △E-A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Li, H.; Li, L.-F.; Zhang, Z.-J.; Wu, C.-J.; Yu, S.-J. Sensory evaluation, chemical structures, and treshold concentrations of bitter-tasting compounds in common foodstuffs derived from plants and maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2021, 20, 1–41. [Google Scholar]
- Ares, A.M.; Tapia, J.A.; González-Porto, A.V.; Higes, M.; Martín-Hernández, R.; Bernal, J. Glucosinolates as Markers of the Origin and Harvesting Period for Discrimination of Bee Pollen by UPLC-MS/MS. Foods 2022, 11, 1446. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and flavour perceptions of glucosinolate, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 2018, 62, 1700990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomic, N.; Dojnov, B.; Miocinovic, J.; Tomasevic, I.; Smigic, M.; Djekic, I.; Vujcic, Z. Enrichment of yoghurt with insoluble dietary fiber from triticale—A sensory perspective. LWT—Food Sci. Technol. 2017, 80, 59–66. [Google Scholar] [CrossRef]


| Ingredient | Amount (g) (Control Sample) | Amount (g) (Enriched Samples) | ||
|---|---|---|---|---|
| 2% Pollen | 5% Pollen | 10% Pollen | ||
| wheat flour | 100.00 | 98.00 | 95.00 | 90.00 |
| pollen | 0.00 | 2.00 | 5.00 | 10.00 |
| ground sugar | 57.80 | 57.80 | 57.80 | 57.80 |
| margarine | 28.40 | 28.40 | 28.40 | 28.40 |
| salt | 0.93 | 0.93 | 0.93 | 0.93 |
| distilled water | 7.11 | 7.11 | 7.11 | 7.11 |
| glucose syrup | 14.60 | 14.60 | 14.60 | 14.60 |
| baking soda | 1.11 | 1.11 | 1.11 | 1.11 |
| Biscuit | Moisture | Carbohydrate | Crude Protein | Crude Fat | Ash |
|---|---|---|---|---|---|
| (g/100 g Sample) | |||||
| control | 4.52 ± 0.12 a | 77.66 ± 0.47 abc | 5.43 ± 0.13 c | 11.35 ± 0.27 ab | 1.05 ± 0.12 b |
| rapeseed 2% | 4.48 ± 0.09 a | 77.64 ± 0.49 abc | 5.58 ± 0.19 abc | 11.21 ± 0.58 ab | 1.09 ± 0.05 b |
| rapeseed 5% | 4.41 ± 0.14 ab | 77.10 ± 0.17 abc | 5.74 ± 0.08 abc | 11.66 ± 0.37 ab | 1.09 ± 0.15 b |
| rapeseed 10% | 4.33 ± 0.14 abc | 76.08 ± 0.39 bc | 6.49 ± 0.30 ab | 11.72 ± 0.17 ab | 1.38 ± 0.04 a |
| phacelia 2% | 4.17 ± 0.13 abc | 77.03 ± 0.63 abc | 5.54 ± 0.24 abc | 12.18 ± 0.35 a | 1.08 ± 0.01 ab |
| phacelia 5% | 3.94 ± 0.05 c | 75.99 ± 0.67 c | 6.08 ± 0.20 abc | 12.83 ± 0.50 a | 1.16 ± 0.02 ab |
| phacelia 10% | 4.01 ± 0.12 bc | 76.24 ± 0.73 bc | 6.56 ± 0.09 a | 12.06 ± 0.65 ab | 1.13 ± 0.07 ab |
| sunflower 2% | 4.37 ± 0.13 ab | 78.06 ± 0.39 ab | 5.56 ± 0.22 abc | 10.91 ± 0.40 b | 1.10 ± 0.04 ab |
| sunflower 5% | 4.22 ± 0.16 abc | 78.45 ± 0.61 a | 5.46 ± 0.24 bc | 10.70 ± 0.66 b | 1.18 ± 0.10 ab |
| sunflower 10% | 4.05 ± 0.13 abc | 77.32 ± 0.43 abc | 5.44 ± 0.18 c | 12.08 ± 0.40 ab | 1.10 ± 0.08 ab |
| Biscuit | TPC (mg GAE/100 g) | FRAP (mg AAE/100 g) | TEAC (mg TE/100 g) | DPPH (mg TE/100 g) |
|---|---|---|---|---|
| control | 50.53 ± 1.61 d | 13.42 ± 1.46 d | 21.17 ± 1.68 c | 11.66 ± 2.75 c |
| rapeseed 2% | 57.54 ± 2.00 cd | 18.45 ± 0.86 cd | 22.77 ± 1.14 bc | 24.33 ± 2.77 bc |
| rapeseed 5% | 71.49 ± 1.29 abcd | 32.42 ± 2.28 abcd | 31.61 ± 1.21 abc | 49.74 ± 3.04 abc |
| rapeseed 10% | 98.13 ± 3.02 ab | 46.51 ± 2.51 abc | 42.18 ± 1.61 ab | 68.52 ± 6.31 ab |
| phacelia 2% | 65.63 ± 1.62 abcd | 21.86 ± 1.69 bcd | 27.14 ± 1.24 abc | 36.97 ± 1.82 abc |
| phacelia 5% | 90.83 ± 5.39 abc | 41.47 ± 2.64 abcd | 36.72 ± 0.94 abc | 75.51 ± 1.16 a |
| phacelia 10% | 123.66 ± 2.15 a | 63.54 ± 2.16 a | 52.92 ± 0.91 a | 104.25 ± 1.53 a |
| sunflower 2% | 60.22 ± 1.36 cd | 23.25 ± 0.82 bcd | 21.42 ± 1.74 c | 26.54 ± 6.49 bc |
| sunflower 5% | 75.88 ± 2.65 abcd | 37.57 ± 2.03 abcd | 34.63 ± 0.96 abc | 58.35 ± 1.90 abc |
| sunflower 10% | 93.46 ± 1.14 abc | 57.81 ± 1.64 ab | 42.60 ± 1.13 a | 70.51 ± 2.39 ab |
| Biscuit | L* | a* | b* | C* | h° |
|---|---|---|---|---|---|
| control | 70.3 ± 0.72 a | 1.0 ± 0.48 f | 31.8 ± 0.58 f | 31.8 ± 0.59 g | 88.2 ± 0.84 a |
| rapeseed 2% | 67.4 ± 0.94 b | 4.5 ± 0.63 e | 32.9 ± 0.32 e | 33.2 ± 0.37 f | 82.3 ± 1.04 b |
| rapeseed 5% | 64.4 ± 1.01 c | 5.9 ± 0.56 d | 34.2 ± 0.34 d | 34.7 ± 0.40 e | 80.2 ± 0.86 d |
| rapeseed 10% | 60.9 ± 1.38 d | 7.3 ± 0.70 bc | 35.3 ± 0.34 c | 36.1 ± 0.31 c | 78.3 ± 1.13 e |
| phacelia 2% | 61.6 ± 0.74 d | 4.6 ± 0.52 e | 29.6 ± 0.39 g | 30.0 ± 0.44 h | 81.2 ± 0.92 c |
| phacelia 5% | 55.9 ± 1.36 f | 6.9 ± 0.43 c | 27.6 ± 0.47 h | 28.5 ± 0.55 i | 75.9 ± 0.65 f |
| phacelia 10% | 50.1 ± 1.36 g | 9.1 ± 0.47 a | 26.7 ± 0.67 i | 28.2 ± 0.68 i | 71.2 ± 0.92 g |
| sunflower 2% | 68.0 ± 0.87 b | 4.6 ± 0.57 e | 34.9 ± 0.76 c | 35.2 ± 0.73 d | 82.5 ± 0.98 b |
| sunflower 5% | 61.3 ± 0.98 d | 7.9 ± 0.47 b | 36.8 ± 0.53 b | 37.7 ± 0.54 b | 77.8 ± 0.70 e |
| sunflower 10% | 59.5 ± 0.89 e | 8.8 ± 0.68 a | 40.5 ± 0.81 a | 41.5 ± 0.86 a | 77.7 ± 0.87 e |
| Biscuit | Control | Rapeseed 2% | Rapeseed 5% | Rapeseed 10% | Phacelia 2% | Phacelia 5% | Phacelia 10% | Sunflower 2% | Sunflower 5% | Sunflower 10% |
|---|---|---|---|---|---|---|---|---|---|---|
| control | 4.6 | 8.0 | 11.9 | 9.6 | 16.1 | 22.3 | 5.3 | 12.4 | 15.9 | |
| rapeseed 2% | 3.6 | 7.5 | 6.7 | 12.9 | 19.0 | 2.1 | 8.1 | 11.8 | ||
| rapeseed 5% | 3.9 | 5.5 | 10.8 | 16.4 | 3.9 | 4.6 | 8.5 | |||
| rapeseed 10% | 6.4 | 9.2 | 13.9 | 7.6 | 1.7 | 5.6 | ||||
| phacelia 2% | 6.5 | 12.7 | 8.3 | 8.0 | 11.9 | |||||
| phacelia 5% | 6.3 | 14.3 | 10.7 | 13.5 | ||||||
| phacelia 10% | 20.2 | 15.1 | 16.7 | |||||||
| sunflower 2% | 7.8 | 11.1 | ||||||||
| sunflower 5% | 4.2 | |||||||||
| sunflower 10% |
| Biscuit | Diameter (mm) | Height (mm) | Volume (cm3) | Area (cm2) | Density (g/cm3) | Baking Loss (%) |
|---|---|---|---|---|---|---|
| control | 60 ± 0.96 ab | 9 ± 0.96 ab | 24.60 ± 3.51 ab | 28.04 ± 0.90 ab | 0.53 ± 0.10 ab | 14.78 ± 1.60 ab |
| rapeseed 2% | 59 ± 2.22 ab | 9 ± 0.50 ab | 23.76 ± 2.50 ab | 27.14 ± 2.04 ab | 0.54 ± 0.05 ab | 13.92 ± 1.65 ab |
| rapeseed 5% | 57 ± 1.71 ab | 8 ± 0.00 ab | 20.25 ± 1.22 ab | 25.31 ± 1.53 ab | 0.64 ± 0.04 ab | 15.58 ± 3.33 ab |
| rapeseed 10% | 58 ± 1.29 ab | 8 ± 0.50 a | 20.14 ± 1.77 ab | 25.30 ± 0.86 ab | 0.67 ± 0.06 b | 12.94 ± 0.18 a |
| phacelia 2% | 55 ± 0.50 a | 9 ± 0.58 ab | 20.39 ± 1.63 ab | 23.98 ± 0.44 a | 0.63 ± 0.07 ab | 15.72 ± 0.32 b |
| phacelia 5% | 56 ± 0.96 a | 8 ± 0.00 ab | 19.53 ± 0.67 a | 24.42 ± 0.84 a | 0.64 ± 0.02 ab | 13.76 ± 0.36 ab |
| phacelia 10% | 58 ± 0.50 ab | 8 ± 0.00 ab | 20.96 ± 0.36 ab | 26.20 ± 0.45 ab | 0.60 ± 0.02 ab | 16.13 ± 0.60 b |
| sunflower 2% | 59 ± 0.96 ab | 10 ± 0.58 b | 26.21 ± 2.00 b | 26.90 ± 1.75 ab | 0.49 ± 0.05 ab | 14.68 ± 0.51 ab |
| sunflower 5% | 62 ± 0.58 b | 9 ± 0.50 ab | 25.98 ± 1.29 b | 29.71 ± 0.56 b | 0.47 ± 0.04 a | 13.79 ± 0.53 ab |
| sunflower 10% | 59 ± 1.41 ab | 8 ± 0.50 ab | 22.59 ± 2.11 ab | 26.96 ± 1.56 ab | 0.55 ± 0.05 ab | 14.70 ± 0.40 ab |
| Biscuit | Hardness (g) | Adhesive Force (g) | Fracturability (g) | Quantity of Fractures | Cohesiveness | Springiness (mm) | Guminess (g) | Chewiness (mJ) |
|---|---|---|---|---|---|---|---|---|
| control | 454 ± 108.14 bcd | 80 ± 14.67 abc | 170 ± 80.75 abc | 7 ± 1.58 a | 0.13 ± 0.01 a | 1.36 ± 0.14 ab | 64 ± 17.89 bcd | 1.05 ± 0.23 bcde |
| rapeseed 2% | 472 ± 114.78 bcd | 131 ± 24.16 c | 176 ± 25.37 abc | 8 ± 2.05 a | 0.13 ± 0.01 a | 2.22 ± 0.58 bc | 66 ± 10.22 bcd | 1.40 ± 0.33 cde |
| rapeseed 5% | 713 ± 107.96 d | 130 ± 56.80 abc | 263 ± 121.44 bc | 7 ± 1.73 a | 0.14 ± 0.01 ab | 2.08 ± 0.95 abc | 99 ± 9.70 d | 2.18 ± 0.87 de |
| rapeseed 10% | 269 ± 93.21 ab | 66 ± 30.64 ab | 121 ± 10.41 a | 6 ± 1.48 a | 0.16 ± 0.03 b | 1.88 ± 0.55 ab | 41 ± 9.29 abc | 0.87 ± 0.43 abcd |
| phacelia 2% | 620 ± 129.13 cd | 183 ± 78.22 c | 268 ± 81.45 bc | 7 ± 1.32 a | 0.13 ± 0.02 ab | 1.41 ± 0.18 ab | 72 ± 11.37 cd | 1.07 ± 0.34 bcde |
| phacelia 5% | 242 ± 86.61 ab | 89 ± 28.48 abc | 154 ± 27.62 ab | 6 ± 2.03 a | 0.14 ± 0.01 ab | 1.52 ± 0.25 abc | 30 ± 4.45 ab | 0.55 ± 0.16 ab |
| phacelia 10% | 875 ± 235.37 d | 136 ± 43.62 bc | 294 ± 43.30 c | 7 ± 1.00 a | 0.12 ± 0.01 a | 3.49 ± 0.83 c | 105 ± 12.14 d | 2.96 ± 0.60 e |
| sunflower 2% | 283 ± 78.64 abc | 91 ± 30.25 abc | 190 ± 38.17 abc | 6 ± 1.73 a | 0.13 ± 0.01 ab | 1.39 ± 0.30 a | 34 ± 7.44 abc | 0.52 ± 0.10 ab |
| sunflower 5% | 258 ± 11.99 abc | 68 ± 27.18 ab | 168 ± 48.81 abc | 6 ± 0.71 a | 0.13 ± 0.02 ab | 1.60 ± 0.28 abc | 30 ± 5.41 ab | 0.58 ± 0.14 abc |
| sunflower 10% | 196 ± 38.25 a | 64 ± 12.21 a | 155 ± 16.50 ab | 5 ± 1.50 a | 0.14 ± 0.01 ab | 1.49 ± 0.14 ab | 27 ± 5.07 a | 0.39 ± 0.08 a |
| Attribute | F-Prod | F-Scal | F-Disag | RMSE |
|---|---|---|---|---|
| yellow colour intensity | 1571.13 | 0.92 | 0.85 | 2.54 |
| red colour intensity | 1232.00 | 0.96 | 0.92 | 1.66 |
| cabbage flavour intensity | 1141.42 | 2.04 * | 0.97 | 1.43 |
| cut hay flavour intensity | 1128.14 | 3.27 * | 0.54 | 1.29 |
| cut hay odour intensity | 1003.56 | 2.21 * | 0.71 | 1.43 |
| cabbage odour intensity | 877.93 | 4.05 * | 0.56 | 1.96 |
| global odour intensity | 641.21 | 1.81 | 0.76 | 2.37 |
| global flavour intensity | 386.40 | 1.40 | 0.84 | 3.15 |
| flour odour intensity | 340.47 | 0.87 | 1.11 | 2.35 |
| amount of specks on the surface | 289.45 | 0.32 | 0.93 | 0.96 |
| flour flavour intensity | 179.94 | 0.69 | 1.08 | 1.44 |
| adhesiveness | 96.47 | 1.39 | 1.07 | 1.46 |
| fracturability | 67.69 | 0.80 | 1.02 | 2.53 |
| sweet taste intensity | 48.50 | 1.46 | 1.08 | 2.48 |
| sweet odour intensity | 37.25 | 0.78 | 1.08 | 2.90 |
| mouthcoating | 29.30 | 0.37 | 1.17 | 1.87 |
| hardness | 27.99 | 0.80 | 1.08 | 2.21 |
| chewiness | 21.99 | 1.36 | 0.83 | 2.03 |
| crumbliness | 21.63 | 0.57 | 1.22 | 1.48 |
| margarine odour intensity | 14.14 | 0.83 | 0.64 | 2.86 |
| brightness | 9.62 | 4.80 * | 0.69 | 46.88 * |
| bitter taste intensity | 6.99 | 0.63 | 0.97 | 1.48 |
| margarine flavour intensity | 6.24 | 0.71 | 1.33 | 1.19 |
| salt taste intensity | 5.90 | 0.83 | 0.77 | 1.29 |
| shape regularity | 5.84 | 2.08 * | 0.91 | 0.79 |
| bitter odour intensity | 1.39 * | 0.98 | 0.79 | 1.76 |
| baking soda flavour intensity | 1.38 * | 0.61 | 0.54 | 1.81 |
| salt odour intensity | 0.98 * | 1.55 | 0.63 | 1.20 |
| sour taste intensity | 0.76 * | 1.78 | 0.90 | 1.31 |
| baking soda odour intensity | 0.60 * | 0.35 | 0.93 | 1.85 |
| sour odour intensity | 0.26 * | 0.31 | 1.14 | 1.71 |
| Sensory Attributes | Biscuit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Rapeseed 2% | Rapeseed 5% | Rapeseed 10% | Phacelia 2% | Phacelia 5% | Phacelia 10% | Sunflower 2% | Sunflower 5% | Sunflower 10% | |
| shape regularity | 95.0 ± 0.9 a | 94.3 ± 0.7 b | 94.1 ± 0.9 b | 93.9 ± 0.7 b | 94.1 ± 0.8 b | 94.2 ± 0.6 b | 94.0 ± 0.7 b | 93.7 ± 0.8 b | 93.8 ± 0.8 b | 93.9 ± 0.8 b |
| homogeneity of the surface | 81.8 ± 2.1 d | 82.5 ± 1.7 d | 83.0 ± 1.7 d | 91.1 ± 2.8 b | 82.4 ± 1.4 d | 93.9 ± 1.2 a | 94.5 ± 1.3 a | 82.0 ± 1.6 d | 85.8 ± 1.4 c | 93.7 ± 1.6 a |
| amount of specks on the surface | 0.0 ± 0.0 d | 2.3 ± 1.2 c | 4.4 ± 0.9 b | 9.1 ± 1.2 a | 3.0 ± 0.8 c | 4.6 ± 0.9 b | 9.3 ± 0.9 a | 2.6 ± 0.9 c | 4.5 ± 0.8 b | 8.9 ± 1.0 a |
| lightness | 88.3 ± 2.2 a | 79.2 ± 1.7 b | 75.1 ± 1.1 c | 68.4 ± 2.1 d | 49.7 ± 2.2 f | 38.7 ± 2.5 g | 24.5 ± 2.3 h | 80.3 ± 1.4 b | 67.1 ± 2.0 d | 60.8 ± 2.6 e |
| red colour intensity | 4.5 ± 1.4 f | 5.0 ± 1.4 ef | 6.1 ± 1.6 de | 7.5 ± 2.0 cd | 5.1 ± 1.3 ef | 6.0 ± 1.3 ef | 6.0 ± 1.3 ef | 8.5 ± 1.5 c | 23.8 ± 2.2 b | 39.2 ± 2.0 a |
| yellow colour intensity | 40.9 ± 2.3 d | 51.4 ± 2.2 c | 61.9 ± 2.0 b | 80.9 ± 4.5 a | 31.5 ± 2.9 e | 24.2 ± 1.6 f | 15.7 ± 1.1 g | 50.6 ± 2.0 c | 51.4 ± 2.3 c | 52.7 ± 2.9 c |
| overall odour intensity | 54.2 ± 2.6 d | 60.5 ± 2.0 c | 69.5 ± 2.3 b | 82.9 ± 2.1 a | 60.2 ± 1.9 c | 70.2 ± 2.7 b | 82.3 ± 2.3 a | 59.2 ± 1.9 c | 70.5 ± 2.8 b | 82.5 ± 2.2 a |
| flour odour intensity | 59.8 ± 2.3 a | 55.5 ± 2.3 b | 51.6 ± 2.6 c | 42.7 ± 2.2 d | 54.5 ± 2.5 b | 51.1 ± 2.4 c | 32.5 ± 2.2 e | 55.7 ± 2.6 b | 50.9 ± 2.3 c | 33.9 ± 2.5 e |
| margarine odour intensity | 21.8 ± 2.4 a | 21.3 ± 2.6 ab | 22.2 ± 2.3 a | 19.1 ± 3.3 c | 21.7 ± 2.6 a | 19.8 ± 2.6 bc | 17.0 ± 1.9 d | 22.0 ± 2.1 a | 19.1 ± 1.8 c | 18.7 ± 3.5 c |
| baking soda odour intensity | 1.6 ± 1.9 a | 2.0 ± 2.0 a | 1.5 ± 1.7 a | 1.7 ± 2.0 a | 1.8 ± 2.0 a | 1.6 ± 1.6 a | 1.6 ± 1.2 a | 1.0 ± 1.6 a | 1.9 ± 1.9 a | 1.5 ± 1.8 a |
| sweet odour intensity | 53.8 ± 2.9 a | 50.5 ± 3.1 bc | 44.4 ± 2.8 d | 43.9 ± 2.8 d | 52.0 ± 2.2 ab | 50.0 ± 3.4 bc | 44.2 ± 2.9 d | 49.0 ± 3.1 c | 45.7 ± 3.2 d | 43.7 ± 2.9 d |
| sour odour intensity | 1.8 ± 1.6 a | 1.5 ± 1.8 a | 1.6 ± 1.6 a | 1.6 ± 2.0 a | 1.8 ± 1.8 a | 1.6 ± 1.5 a | 1.7 ± 1.5 a | 1.3 ± 1.6 a | 1.6 ± 1.8 a | 2.0 ± 2.1 a |
| bitter odour intensity | 0.4 ± 1.0 a | 0.5 ± 1.2 a | 0.8 ± 1.5 a | 1.1 ± 1.9 a | 0.6 ± 1.3 a | 1.2 ± 2.1 a | 1.5 ± 2.3 a | 0.8 ± 1.4 a | 1.0 ± 1.7 a | 1.4 ± 2.2 a |
| salt odour intensity | 0.4 ± 0.9 a | 0.4 ± 0.8 a | 0.6 ± 1.1 a | 0.7 ± 1.3 a | 0.7 ± 1.3 a | 0.8 ± 1.5 a | 0.8 ± 1.5 a | 0.3 ± 0.7 a | 0.5 ± 1.1 a | 0.5 ± 1.3 a |
| cut hay odour intensity | 0.3 ± 0.8 d | 0.3 ± 0.8 d | 0.5 ± 0.9 d | 0.7 ± 1.3 d | 10.4 ± 1.6 c | 15.5 ± 2.2 b | 21.3 ± 2.3 a | 0.5 ± 0.9 d | 0.5 ± 1.0 d | 0.5 ± 1.1 d |
| cabbage odour intensity | 0.0 ± 0.0 d | 9.0 ± 1.7 c | 17.6 ± 3.0 b | 24.9 ± 3.1 a | 0.6 ± 1.4 d | 0.8 ± 1.7 d | 1.0 ± 1.8 d | 0.3 ± 0.9 d | 0.4 ± 1.1 d | 0.6 ± 1.5 d |
| overall flavour intensity | 59.9 ± 3.0 d | 60.0 ± 3.8 d | 73.4 ± 3.0 c | 84.0 ± 2.3 b | 60.6 ± 3.6 d | 72.4 ± 2.9 c | 90.9 ± 2.8 a | 61.0 ± 3.5 d | 71.2 ± 2.9 c | 84.8 ± 2.6 b |
| flour flavour intensity | 17.4 ± 2.1 a | 12.7 ± 1.8 b | 9.3 ± 1.6 c | 4.8 ± 0.8 d | 12.2 ± 1.6 b | 10.0 ± 1.2 c | 4.8 ± 1.0 d | 11.9 ± 1.6 b | 10.0 ± 1.6 c | 4.9 ± 0.7 d |
| margarine flavour intensity | 10.5 ± 1.2 ab | 10.8 ± 1.4 a | 10.7 ± 1.1 ab | 9.8 ± 1.4 abc | 10.0 ± 1.3 abc | 9.5 ± 1.7 bc | 9.0 ± 1.0 c | 10.5 ± 1.4 ab | 9.0 ± 0.9 c | 9.2 ± 1.2 c |
| baking soda flavour intensity | 1.8 ± 1.8 a | 1.3 ± 1.5 a | 1.3 ± 1.6 a | 2.0 ± 2.1 a | 1.3 ± 1.6 a | 1.1 ± 1.2 a | 1.2 ± 1.4 a | 1.1 ± 1.4 a | 1.7 ± 1.7 a | 1.0 ± 1.4 a |
| sweet taste intensity | 55.0 ± 1.8 a | 50.2 ± 2.9 b | 44.0 ± 2.4 cd | 43.2 ± 2.3 d | 48.2 ± 3.3 b | 48.9 ± 1.3 b | 45.5 ± 3.8 c | 49.5 ± 2.4 b | 45.1 ± 2.3 cd | 44.1 ± 2.3 cd |
| sour taste intensity | 1.3 ± 1.2 a | 1.2 ± 1.4 a | 1.4 ± 1.3 a | 0.9 ± 1.2 a | 1.6 ± 1.4 a | 1.1 ± 1.3 a | 1.3 ± 1.1 a | 0.9 ± 1.1 a | 1.3 ± 1.4 a | 1.3 ± 1.5 a |
| bitter taste intensity | 0.4 ± 1.0 d | 1.3 ± 1.4 bcd | 1.6 ± 1.7 abcd | 2.5 ± 2.1 ab | 1.1 ± 1.1 bcd | 1.9 ± 1.5 abc | 2.8 ± 1.6 a | 0.9 ± 1.1 cd | 1.6 ± 1.5 abcd | 2.3 ± 1.6 ab |
| salt taste intensity | 1.0 ± 1.0 c | 1.0 ± 1.1 c | 1.3 ± 1.3 bc | 2.3 ± 1.2 ab | 1.5 ± 1.4 abc | 1.7 ± 1.4 abc | 2.4 ± 1.1 ab | 1.5 ± 1.4 abc | 1.9 ± 1.2 abc | 2.5 ± 1.1 a |
| cut hay flavour intensity | 0.4 ± 0.8 d | 0.6 ± 0.9 d | 0.6 ± 0.9 d | 0.6 ± 0.9 d | 9.8 ± 1.1 c | 13.4 ± 1.8 b | 17.1 ± 2.2 a | 0.5 ± 0.8 d | 0.5 ± 0.9 d | 0.5 ± 0.8 d |
| cabbage flavour intensity | 0.0 ± 0.0 d | 9.2 ± 1.3 c | 18.9 ± 1.7 b | 27.6 ± 2.5 a | 0.6 ± 0.9 d | 0.8 ± 1.2 d | 0.9 ± 1.2 d | 0.3 ± 0.8 d | 0.3 ± 0.8 d | 0.7 ± 1.0 d |
| hardness | 33.3 ± 2.5 a | 31.8 ± 2.3 ab | 28.3 ± 1.8 de | 26.1 ± 2.0 f | 31.0 ± 2.2 bc | 29.0 ± 2.4 cd | 26.4 ± 2.7 ef | 31.2 ± 2.0 b | 29.0 ± 2.2 cd | 26.7 ± 1.5 ef |
| chewiness | 22.4 ± 1.8 d | 22.3 ± 1.5 d | 23.8 ± 2.5 cd | 25.6 ± 1.6 ab | 22.0 ± 2.3 d | 24.4 ± 2.1 bc | 26.7 ± 2.3 a | 22.2 ± 1.9 d | 24.7 ± 1.9 bc | 26.2 ± 1.6 ab |
| adhesiveness | 5.7 ± 1.2 e | 8.8 ± 0.9 cd | 10.1 ± 1.1 bc | 15.4 ± 1.7 a | 9.1 ± 0.9 bcd | 10.0 ± 1.3 bcd | 14.6 ± 2.5 a | 8.7 ± 1.1 d | 10.4 ± 1.1 b | 14.0 ± 2.3 a |
| mouthcoating | 16.3 ± 1.2 c | 19.1 ± 1.7 b | 19.5 ± 1.8 b | 23.6 ± 1.6 a | 19.0 ± 2.4 b | 19.0 ± 1.9 b | 22.9 ± 2.5 a | 19.6 ± 1.6 b | 19.1 ± 1.7 b | 22.5 ± 2.1 a |
| crumbliness | 19.5 ± 1.7 a | 16.8 ± 1.5 bc | 15.8 ± 1.7 cd | 14.6 ± 1.8 d | 18.0 ± 1.7 b | 15.5 ± 1.7 cd | 15.1 ± 1.2 d | 16.9 ± 1.4 bc | 15.3 ± 1.3 d | 15.1 ± 1.2 d |
| fracturability | 53.4 ± 2.4 a | 46.5 ± 3.1 c | 44.5 ± 2.2 c | 42.1 ± 2.1 d | 49.6 ± 2.0 b | 46.6 ± 3.6 c | 39.9 ± 2.3 d | 49.0 ± 2.2 b | 48.9 ± 2.6 b | 41.5 ± 1.8 d |
| Sensory Attributes | Consumer Groups | Biscuit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Rapeseed 2% | Rapeseed 5% | Rapeseed 10% | Phacelia 2% | Phacelia 5% | Phacelia 10% | Sunflower 2% | Sunflower 5% | Sunflower 10% | ||
| colour liking | cluster 1 | 5.6 ± 2.2 abc | 6.5 ± 1.3 ab | 6.3 ± 1.9 ab | 6.8 ± 1.6 ab | 6.4 ± 1.6 ab | 4.3 ± 1.8 c | 5.1 ± 2.4 bc | 7.1 ± 1.1 a | 7.3 ± 1.3 a | 6.9 ± 1.9 ab |
| cluster 2 | 5.8 ± 2.1 bc | 6.1 ± 1.7 abc | 7.2 ± 1.6 a | 6.7 ± 1.8 ab | 5.8 ± 2.1 bc | 5.1 ± 1.9 cd | 4.1 ± 2.1 d | 6.7 ± 1.7 ab | 6.7 ± 1.6 ab | 6.8 ± 1.7 ab | |
| cluster 3 | 6.7 ± 1.5 ab | 6.5 ± 1.3 ab | 6.9 ± 1.6 a | 6.7 ± 1.9 ab | 5.6 ± 1.9 bc | 4.6 ± 2.1 c | 5.0 ± 2.3 c | 6.8 ± 1.8 ab | 7.0 ± 1.6 a | 6.4 ± 2.0 ab | |
| all consumers | 6.2 ± 1.9 ab | 6.4 ± 1.4 ab | 6.9 ± 1.7 a | 6.7 ± 1.8 a | 5.8 ± 1.9 b | 4.7 ± 2.0 c | 4.7 ± 2.3 c | 6.8 ± 1.7 a | 6.9 ± 1.6 a | 6.7 ± 1.9 a | |
| overall odour liking | cluster 1 | 6.0 ± 1.5 a | 5.6 ± 2.2 a | 5.1 ± 1.9 a | 5.8 ± 2.6 a | 6.4 ± 1.4 a | 5.6 ± 1.1 a | 5.4 ± 2.2 a | 5.6 ± 1.5 a | 6.8 ± 1.3 a | 6.2 ± 1.8 a |
| cluster 2 | 5.6 ± 1.8 a | 5.8 ± 1.9 a | 6.1 ± 2.0 a | 5.3 ± 2.1 a | 6.0 ± 1.9 a | 5.7 ± 1.7 a | 3.9 ± 1.9 b | 6.3 ± 1.6 a | 5.6 ± 1.7 a | 6.2 ± 2.2 a | |
| cluster 3 | 6.0 ± 1.5 a | 5.6 ± 1.7 a | 5.2 ± 2.0 ab | 3.7 ± 2.3 c | 5.5 ± 1.5 ab | 5.5 ± 1.5 ab | 4.4 ± 1.9 bc | 6.3 ± 1.4 a | 6.3 ± 1.5 a | 5.3 ± 2.3 ab | |
| all consumers | 5.9 ± 1.6 a | 5.7 ± 1.8 a | 5.6 ± 2.0 a | 4.7 ± 2.4 b | 5.8 ± 1.6 a | 5.6 ± 1.5 a | 4.4 ± 2.0 b | 6.2 ± 1.5 a | 6.1 ± 1.6 a | 5.8 ± 2.2 a | |
| sweet odour liking | cluster 1 | 5.5 ± 2.1 a | 5.4 ± 2.2 a | 5.6 ± 1.1 a | 5.2 ± 2.2 a | 5.4 ± 1.7 a | 5.4 ± 1.4 a | 5.0 ± 1.5 a | 5.3 ± 1.4 a | 5.7 ± 1.4 a | 5.6 ± 2.0 a |
| cluster 2 | 5.4 ± 1.6 a | 5.7 ± 1.6 a | 6.2 ± 2.0 a | 5.0 ± 2.1 ab | 5.6 ± 2.0 a | 5.6 ± 1.6 a | 3.8 ± 1.7 b | 5.6 ± 1.6 a | 5.2 ± 1.7 a | 5.8 ± 1.9 a | |
| cluster 3 | 5.8 ± 2.2 a | 5.4 ± 2.1 ab | 5.4 ± 2.0 ab | 4.0 ± 2.3 c | 5.4 ± 1.6 ab | 5.1 ± 1.7 abc | 4.2 ± 2.0 bc | 5.9 ± 1.8 a | 6.3 ± 1.7 a | 5.1 ± 2.0 abc | |
| all consumers | 5.6 ± 1.9 a | 5.5 ± 1.9 a | 5.8 ± 1.9 a | 4.6 ± 2.2 bc | 5.5 ± 1.8 a | 5.4 ± 1.6 ab | 4.2 ± 1.9 c | 5.7 ± 1.7 a | 5.8 ± 1.7 a | 5.5 ± 2.0 a | |
| margarine odour liking | cluster 1 | 5.0 ± 1.2 a | 5.1 ± 1.8 a | 5.8 ± 1.1 a | 5.2 ± 1.7 a | 6.0 ± 1.3 a | 5.5 ± 1.9 a | 4.9 ± 1.3 a | 5.4 ± 1.4 a | 5.8 ± 1.5 a | 5.1 ± 1.4 a |
| cluster 2 | 5.5 ± 1.1 ab | 5.5 ± 1.4 ab | 5.6 ± 1.6 ab | 4.8 ± 1.9 bc | 5.6 ± 1.8 ab | 5.7 ± 1.5 ab | 4.2 ± 1.7 c | 6.0 ± 1.4 a | 5.1 ± 1.5 abc | 5.7 ± 1.6 ab | |
| cluster 3 | 5.4 ± 1.4 ab | 5.3 ± 1.7 ab | 5.2 ± 1.6 ab | 4.0 ± 2.0 c | 5.1 ± 1.6 abc | 5.1 ± 1.6 abc | 4.6 ± 1.8 bc | 5.9 ± 1.5 a | 5.6 ± 1.5 ab | 5.4 ± 1.5 ab | |
| all consumers | 5.4 ± 1.3 a | 5.4 ± 1.6 a | 5.4 ± 1.5 a | 4.5 ± 1.9 b | 5.4 ± 1.7 a | 5.4 ± 1.6 a | 4.5 ± 1.7 b | 5.9 ± 1.4 a | 5.4 ± 1.5 a | 5.5 ± 1.5 a | |
| overall flavour liking | cluster 1 | 6.8 ± 1.0 a | 6.1 ± 1.8 a | 5.4 ± 2.2 a | 7.1 ± 1.8 a | 6.8 ± 1.6 a | 5.5 ± 1.6 a | 5.2 ± 2.4 a | 7.2 ± 1.1 a | 7.0 ± 1.3 a | 6.4 ± 2.6 a |
| cluster 2 | 6.2 ± 2.0 abc | 5.1 ± 2.2 c | 6.5 ± 1.7 ab | 5.4 ± 2.4 bc | 7.0 ± 1.5 a | 7.3 ± 1.3 a | 3.0 ± 1.9 d | 7.1 ± 1.3 a | 6.5 ± 1.8 ab | 6.8 ± 1.6 a | |
| cluster 3 | 6.7 ± 1.7 ab | 6.2 ± 2.1 abc | 5.4 ± 1.7 cd | 3.1 ± 1.9 e | 6.5 ± 2.1 abc | 6.4 ± 1.5 abc | 4.7 ± 2.5 d | 6.8 ± 1.5 a | 7.2 ± 1.4 a | 5.4 ± 2.2 bcd | |
| all consumers | 6.5 ± 1.8 ab | 5.8 ± 2.2 b | 5.8 ± 1.8 b | 4.6 ± 2.6 c | 6.7 ± 1.8 a | 6.6 ± 1.6 ab | 4.1 ± 2.42 c | 7.0 ± 1.4 a | 6.9 ± 1.6 a | 6.1 ± 2.2 ab | |
| sweet taste liking | cluster 1 | 6.7 ± 1.6 a | 7.1 ± 1.0 a | 6.3 ± 2.3 a | 7.1 ± 1.9 a | 6.7 ± 1.3 a | 5.9 ± 1.4 a | 6.3 ± 1.7 a | 7.4 ± 1.1 a | 7.3 ± 1.3 a | 6.3 ± 1.6 a |
| cluster 2 | 6.0 ± 1.8 ab | 5.9 ± 2.0 ab | 6.7 ± 1.7 ab | 5.6 ± 2.3 b | 6.6 ± 1.7 ab | 7.1 ± 1.2 a | 3.6 ± 2.2 c | 7.0 ± 1.1 a | 6.1 ± 1.8 ab | 6.6 ± 1.9 ab | |
| cluster 3 | 7.0 ± 1.2 a | 6.3 ± 1.9 ab | 6.1 ± 1.7 ab | 4.2 ± 2.2 d | 6.4 ± 1.8 ab | 6.4 ± 1.7 ab | 4.8 ± 2.3 cd | 6.7 ± 1.6 a | 7.2 ± 1.3 a | 5.5 ± 1.9 bc | |
| all consumers | 6.6 ± 1.6 ab | 6.3 ± 1.9 ab | 6.4 ± 1.8 ab | 5.2 ± 2.4 cd | 6.5 ± 1.7 ab | 6.6 ± 1.5 ab | 4.6 ± 2.4 d | 6.9 ± 1.4 a | 6.8 ± 1.6 ab | 6.0 ± 1.9 bc | |
| margarine flavour liking | cluster 1 | 6.1 ± 1.8 a | 5.8 ± 1.8 a | 5.2 ± 1.5 a | 5.6 ± 1.6 a | 6.1 ± 1.2 a | 5.3 ± 1.5 a | 5.5 ± 1.8 a | 6.8 ± 1.5 a | 6.6 ± 1.4 a | 5.6 ± 2.3 a |
| cluster 2 | 5.6 ± 1.7 ab | 5.2 ± 1.7 b | 5.8 ± 1.5 ab | 5.6 ± 2.1 ab | 6.5 ± 1.4 a | 6.2 ± 1.9 ab | 3.6 ± 1.8 c | 6.4 ± 1.2 ab | 5.9 ± 1.4 ab | 6.2 ± 1.8 ab | |
| cluster 3 | 6.0 ± 1.9 ab | 5.3 ± 1.8 abc | 5.0 ± 1.7 bcd | 4.0 ± 1.8 d | 5.9 ± 1.8 ab | 5.5 ± 2.0 abc | 4.5 ± 1.7 cd | 6.2 ± 1.8 ab | 6.2 ± 1.5 a | 5.0 ± 1.6 bcd | |
| all consumers | 5.8 ± 1.8 abc | 5.4 ± 1.8 cd | 5.4 ± 1.7 cd | 4.9 ± 2.0 de | 6.2 ± 1.6 ab | 5.7 ± 1.9 abc | 4.3 ± 1.9 e | 6.4 ± 1.5 a | 6.2 ± 1.5 ab | 5.6 ± 1.9 bcd | |
| hardness liking | cluster 1 | 5.1 ± 1.8 a | 5.9 ± 2.2 a | 4.6 ± 2.2 a | 5.5 ± 2.6 a | 5.9 ± 2.2 a | 4.1 ± 2.2 a | 5.3 ± 2.6 a | 6.2 ± 2.1 a | 5.6 ± 2.1 a | 5.4 ± 2.3 a |
| cluster 2 | 4.6 ± 2.3 b | 5.7 ± 2.1 ab | 5.0 ± 2.4 b | 4.5 ± 2.6 b | 5.7 ± 2.6 ab | 4.5 ± 2.4 b | 2.6 ± 1.9 c | 6.8 ± 1.9 a | 4.9 ± 2.5 b | 4.6 ± 2.6 b | |
| cluster 3 | 5.1 ± 2.4 abc | 6.3 ± 1.8 ab | 5.1 ± 2.1 bc | 4.3 ± 2.5 c | 5.3 ± 2.3 abc | 4.4 ± 2.4 c | 3.8 ± 2.6 c | 6.6 ± 2.1 a | 6.2 ± 1.9 ab | 5.0 ± 2.5 bc | |
| all consumers | 4.9 ± 2.3 cde | 6.0 ± 2.0 ab | 5.0 ± 2.2 cde | 4.6 ± 2.6 def | 5.6 ± 2.4 bcd | 4.4 ± 2.4 ef | 3.6 ± 2.5 f | 6.6 ± 2.0 a | 5.6 ± 2.2 abc | 4.9 ± 2.5 cde | |
| crumbliness liking | cluster 1 | 6.2 ± 1.8 a | 6.3 ± 2.1 a | 5.9 ± 1.6 a | 5.4 ± 2.0 a | 5.8 ± 1.6 a | 4.8 ± 1.9 a | 6.5 ± 1.9 a | 6.4 ± 2.0 a | 6.3 ± 1.8 a | 5.8 ± 2.0 a |
| cluster 2 | 4.9 ± 2.1 b | 5.9 ± 1.8 ab | 5.8 ± 2.3 ab | 5.8 ± 2.7 ab | 6.0 ± 2.4 ab | 5.7 ± 2.4 ab | 3.8 ± 2.3 c | 6.2 ± 1.8 a | 5.6 ± 2.1 ab | 5.7 ± 2.3 ab | |
| cluster 3 | 6.0 ± 2.0 ab | 6.6 ± 1.9 a | 5.6 ± 1.9 ab | 4.9 ± 2.1 b | 5.2 ± 2.1 b | 5.4 ± 1.9 ab | 5.2 ± 2.1 b | 6.6 ± 1.9 a | 6.6 ± 1.8 a | 5.4 ± 1.8 ab | |
| all consumers | 5.6 ± 2.1 abc | 6.3 ± 1.9 ab | 5.7 ± 2.0 abc | 5.4 ± 2.3 bc | 5.6 ± 2.2 abc | 5.4 ± 2.1 bc | 4.9 ± 2.3 c | 6.4 ± 1.9 a | 6.2 ± 2.0 ab | 5.6 ± 2.0 abc | |
| overall liking | cluster 1 | 6.6 ± 1.4 a | 6.3 ± 1.7 a | 5.4 ± 2.4 ab | 7.1 ± 1.9 a | 7.3 ± 1.1 a | 3.9 ± 2.0 b | 5.9 ± 2.3 ab | 6.4 ± 1.5 a | 6.6 ± 1.4 a | 5.7 ± 2.0 ab |
| cluster 2 | 5.8 ± 2.0 bc | 5.3 ± 2.2 c | 6.7 ± 1.6 ab | 5.7 ± 2.4 bc | 6.6 ± 1.7 abc | 6.4 ± 1.7 abc | 2.5 ± 1.4 d | 7.2 ± 1.4 a | 5.9 ± 2.0 abc | 6.8 ± 1.7 ab | |
| cluster 3 | 6.6 ± 1.7 ab | 6.4 ± 2.3 ab | 5.6 ± 1.8 bc | 3.0 ± 1.7 e | 6.2 ± 1.9 abc | 5.9 ± 1.8 abc | 4.3 ± 2.3 d | 6.9 ± 1.8 a | 7.1 ± 1.7 a | 5.0 ± 2.1 cd | |
| all consumers | 6.3 ± 1.8 ab | 5.9 ± 2.2 b | 6.0 ± 1.9 b | 4.7 ± 2.6 c | 6.5 ± 1.7 ab | 5.8 ± 2.0 b | 3.8 ± 2.3 c | 6.9 ± 1.6 a | 6.6 ± 1.8 ab | 5.8 ± 2.1 b | |
| Biscuit | Not Buying (WDNB + WPNB) | Not Sure | Buying (WPB + WDB) |
|---|---|---|---|
| control | 31 (12 + 19) a | 30 b | 39 (31 + 8) bc |
| rapeseed 2% | 30 (12 + 18) a | 26 ab | 44 (26 + 18) bcd |
| rapeseed 5% | 26 (9 + 17) a | 30 b | 44 (33 + 11) bcd |
| rapeseed 10% | 62 (42 + 20) bc | 15 ab | 23 (12 + 11) ab |
| phacelia 2% | 27 (8 + 19) a | 14 ab | 59 (35 + 24) cd |
| phacelia 5% | 39 (15 + 24) ab | 28 ab | 33 (24 + 9) bc |
| phacelia 10% | 83 (63 + 20) c | 7 a | 10 (3 + 7) a |
| sunflower 2% | 16 (7 + 9) a | 16 ab | 68 (36 + 32) d |
| sunflower 5% | 22 (5 + 17) a | 31 b | 47 (33 + 14) bcd |
| sunflower 10% | 35 (18 + 17) ab | 25 ab | 40 (26 + 14) bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Végh, R.; Csóka, M.; Stefanovits-Bányai, É.; Juhász, R.; Sipos, L. Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference. Foods 2023, 12, 18. https://doi.org/10.3390/foods12010018
Végh R, Csóka M, Stefanovits-Bányai É, Juhász R, Sipos L. Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference. Foods. 2023; 12(1):18. https://doi.org/10.3390/foods12010018
Chicago/Turabian StyleVégh, Rita, Mariann Csóka, Éva Stefanovits-Bányai, Réka Juhász, and László Sipos. 2023. "Biscuits Enriched with Monofloral Bee Pollens: Nutritional Properties, Techno-Functional Parameters, Sensory Profile, and Consumer Preference" Foods 12, no. 1: 18. https://doi.org/10.3390/foods12010018