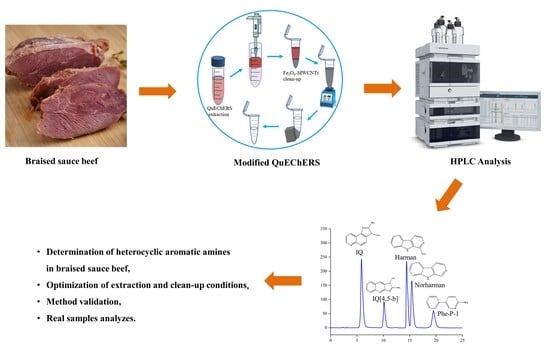
Development of a Modified QuEChERS Method Based on Magnetic Multi-Walled Carbon Nanotubes as a Clean-Up Adsorbent for the Analysis of Heterocyclic Aromatic Amines in Braised Sauce Beef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. HPLC Conditions
2.3. Preparation of Fe3O4-MWCNTs
2.4. Sample Preparation
2.5. Gravimetric Measurement of Co-Extracts
2.6. Method Validation
3. Results and Discussion
3.1. Characterization of Fe3O4-MWCNTs
3.2. Optimization of the Extraction Process
3.2.1. Selection of Extraction Solvent
3.2.2. Selection of the Volume of Water
3.2.3. Selection of the Type of Extraction Salt
3.2.4. Selection of Extraction Time
3.2.5. Selection of Centrifugation Time
3.3. Optimization of the Clean-Up Process
3.3.1. Selection of the Adsorbent
3.3.2. Selection of the Amount of Adsorbent
3.4. Method Validation
3.5. Comparisons with Other Studies
3.6. Analysis of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shan, S.H.; Ma, Y.J.; Sun, C.L.; Guo, X.L.; Zheng, H.; Xu, X.B.; Qin, L.; Hua, J.N. A novel magnetic solid-phase extraction method for detection of 14 heterocyclic aromatic amines by UPLC-MS/MS in meat products. Food Chem. 2021, 337, 127630. [Google Scholar] [CrossRef] [PubMed]
- Śnieżek, E.; Szumska, M.; Nowak, A.; Muzyka, R.; Janoszka, B. Application of high-performance liquid chromatography with fluorescence detection for non-polar heterocyclic aromatic amines and acridine derivatives determination in pork loin roasted in a roasting bag. Foods 2022, 11, 3385. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kotan, G. Effects of different cooking methods and fat levels on the formation of heterocyclic aromatic amines in various fishes. Food Control 2016, 67, 216–224. [Google Scholar] [CrossRef]
- Wang, W.; Dong, L.; Zhang, Y.; Yu, H.N.; Wang, S. Reduction of the heterocyclic amines in grilled beef patties through the combination of thermal food processing techniques without destroying the grilling quality characteristics. Foods 2021, 10, 1490. [Google Scholar] [CrossRef]
- Linghu, Z.; Karim, F.; Taghvaei, M.; Smith, J.S. Determination of heterocyclic amines in meat matrices using enhanced matrix removal-lipid extraction and liquid chromatography-tandem mass spectrometry. J. Food Sci. 2019, 84, 1992–2002. [Google Scholar] [CrossRef]
- Yue, X.Y.; Li, Y.; Xu, S.; Li, J.G.; Li, M.; Jiang, L.Y.; Jie, M.S.; Bai, Y.H. A portable smartphone-assisted ratiometric fluorescence sensor for intelligent and visual detection of malachite green. Food Chem. 2022, 371, 131164. [Google Scholar] [CrossRef]
- Canales, R.; Mariño-Repizo, L.; Reta, M.; Cerutti, S. Multi-response optimization of a green solid-phase extraction for the analysis of heterocyclic aromatic amines in environmental samples. Anal. Methods 2020, 12, 1504. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.X.; Dong, X.W. Determination of heterocyclic amines in braised sauce beef and the effects of different cooking conditions on the formation of heterocyclic amines. J. Sci. Food Agric. 2022, 102, 617–627. [Google Scholar] [CrossRef]
- Yao, G.J.; Zhou, Y.J.; Li, Z.P.; Ma, Q.S. Heterocyclic aromatic amines in roasted chicken: Formation and prediction based on heating temperature and time. Food Chem. 2023, 405, 134822. [Google Scholar] [CrossRef]
- Feng, Y.M.; Xu, Y.; Li, W.R.; Chen, S.S.; Su, Z.Q.; Xi, L.Y.; Li, G.L. Improved enrichment and analysis of heterocyclic aromatic amines in thermally processed foods by magnetic solid phase extraction combined with HPLC-MS/MS. Food Control 2022, 137, 108929. [Google Scholar] [CrossRef]
- Chevolleau, S.; Bouville, A.; Debrauwer, L. Development and validation of a modified QuEChERS protocol coupled to UHPLC-APCI-MS/MS for the simple and rapid quantification of 16 heterocyclic aromatic amines in cooked beef. Food Chem. 2020, 316, 126327. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Chen, B.H.; Kao, T.H. Analysis of heterocyclic amines in meat by the quick, easy, cheap, effective, rugged, and safe method coupled with LC-DAD-MS-MS. J. Agric. Food Chem. 2017, 65, 9360–9368. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.F.; Liao, P.L.; Hsu, K.C.; Shen, J.Y.; Lin, J.L.; Yang, D.J. Establishment of optimal QuEChERS conditions of various food matrices for rapid measurement of heterocyclic amines in various foods. Food Chem. 2022, 380, 132184. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.W.; Lee, Y.T.; Cao, H.; Zhang, H.L.; Chen, B.H. Extraction of heterocyclic amines and polycyclic aromatic hydrocarbons from pork jerky and the effect of flavoring on formation and inhibition. Food Chem. 2023, 402, 134291. [Google Scholar] [CrossRef]
- García, N.G.; Zinola, G.; Macías, G.; Cesio, M.V.; Heinzen, H. Straightforward sample preparation method for the analysis of pesticide residues in ginger (Zingiber officinale Rosc.) using gas chromatography-tandem mass spectrometry. Microchem. J. 2022, 78, 107349. [Google Scholar] [CrossRef]
- Ji, B.C.; Zhao, W.H.; Xu, X.; Han, Y.; Jie, M.S.; Xu, G.G.; Bai, Y.H. Development of a modified quick, easy, cheap, effective, rugged, and safe method based on melamine sponge for multi-residue analysis of veterinary drugs in milks by ultra-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1651, 462333. [Google Scholar] [CrossRef]
- Wang, J.; Duan, H.L.; Fan, L.; Zhang, J.; Zhang, Z.Q. A magnetic fluorinated multi-walled carbon nanotubes-based QuEChERS method for organophosphorus pesticide residues analysis in Lycium ruthenicum Murr. Food Chem. 2021, 338, 127805. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Li, X.Q.; Li, X.J.; Li, X.M.; Li, S.Q.; Zhang, Q.H.; Li, H.M. A functionalized carbon nanotube nanohybrids-based QuEChERS method for detection of pesticide residues in vegetables and fruits. J. Chromatogr. A 2020, 1631, 461526. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.Y.; Han, M.; Qiu, S.T.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhang, Y.W.; Qian, Y.; Peng, Z.Q. Effects of cooking cycle Times of marinating juice and reheating on the formation of cholesterol oxidation products and heterocyclic amines in marinated pig hock. Foods 2020, 9, 1104. [Google Scholar] [CrossRef]
- Yao, Y.; Peng, Z.Q.; Wan, K.H.; Shao, B.; Shi, J.M.; Zhang, Y.W.; Wang, F.L.; Hui, T. Determination of heterocyclic amines in braised sauce beef. Food Chem. 2013, 141, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.H.; Fu, S.C.; Sun, X.Z.; Liu, J.; Zhu, D.Y.; Li, J.H.; Chen, L.X. Magnetic solid-phase extraction using polydopamine-coated magnetic multiwalled carbon nanotube composites coupled with high performance liquid chromatography for the determination of chlorophenols. Analyst 2021, 146, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- SANTE/12682/2019; SANTE Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. European Commission Directorate General for Health and Food Safety Brussels: Brussels, Belgium, 2020.
- Heo, H.M.; Jo, H.W.; Chang, H.R.; Moon, J.K. Development of Simultaneous Analytical Method for Imidazolinone Herbicides from Livestock Products by UHPLC-MSMS. Foods 2022, 11, 1781. [Google Scholar] [CrossRef] [PubMed]
- Freitas, L.V.P.; Alves, L.M.G.; Sicupira, L.C.; Pinho, G.P.; Silvério, F.O. Determination of DDT in honey samples by liquid-liquid extraction with low-temperature purification (LLE-LTP) combined to HPLC-DAD. Anal. Methods 2021, 13, 1955–1964. [Google Scholar] [CrossRef]
- Kemmerich, M.; Rizzetti, T.M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization by central composite design of a modified QuEChERS method for extraction of pesticide multiresidue in sweet pepper and analysis by ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2015, 8, 728–739. [Google Scholar] [CrossRef]
- Hu, M.F.; Qiu, J.S.; Zhang, H.; Fan, X.S.; Liu, K.F.; Zeng, D.Q.; Tan, H.H. Method development and validation of indaziflam and its five metabolites in soil, water, and fruits by modified QuEChERS and UHPLC-MS/MS. J. Agric. Food Chem. 2018, 66, 10300–10308. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, M.M.; Zheng, Z.P.; He, Z.P.; Tao, G.J.; Zhang, S.; Gao, Y.H.; Chen, J. A novel one-step extraction method for simultaneously determining eleven polar heterocyclic aromatic amines in meat products by UHPLC-MS/MS. Anal. Methods 2014, 6, 6437–6444. [Google Scholar] [CrossRef]
- Xing, J.L.; Zhang, Z.G.; Zheng, R.H.; Xu, X.R.; Mao, L.Y.; Lu, J.P.; Shen, J.; Dai, X.J.; Yang, Z.F. Simultaneous detection of seven alternaria toxins in mixed fruit puree by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with a modified QuEChERS. Toxins 2021, 13, 808. [Google Scholar] [CrossRef]
- Tan, X.R.; Jin, Q.; Lu, J.W.; Zhao, B.; Gou, W.N.; Yang, R.; Fu, Y.; Xu, D.H.; Zhang, L. Development and validation of a modified QuEChERS method for the analysis of bisphenols in meats by UPLC-MS/MS. Chromatographia 2022, 85, 433–445. [Google Scholar] [CrossRef]
- Wu, X.Q.; Tong, K.X.; Yu, C.Y.; Hou, S.; Xie, Y.Y.; Fan, C.L.; Chen, H.; Lu, M.L.; Wang, W.W. Development of a high-throughput screening analysis for 195 pesticides in raw milk by modified QuEChERS sample preparation and liquid chromatography quadrupole time-of-flight mass spectrometry. Separations 2022, 9, 98. [Google Scholar] [CrossRef]
- Xu, C.; Liao, Y.Y.; Fang, C.Y.; Tsunoda, M.; Zhang, Y.X.; Song, Y.T.; Deng, S.M. Simultaneous analysis of ursolic acid and oleanolic acid in guava leaves using QuEChERS-based extraction followed by high-performance liquid chromatography. J. Anal. Methods Chem. 2017, 2017, 2984562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of February 23, on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC, Official Journal of the European Union, Strasbourg. 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32017R0978&from=EN (accessed on 16 January 2022).
- Jinap, S.; Mohd-Mokhtar, M.S.; Farhadian, A.; Hasnol, N.D.S.; Jaafar, S.N.; Hajeb, P. Effects of varying degrees of doneness on the formation of Heterocyclic Aromatic Amines in chicken and beef satay. Meat Sci. 2013, 94, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.L.; Quan, W.; He, Z.Y.; Qin, F.; Tao, G.J.; Wang, Z.J.; Zeng, M.M.; Chen, J. Effects of polyphosphates and sodium chloride on heterocyclic amines in roasted beef patties as revealed by UPLC-MS/MS. Food Chem. 2020, 326, 127016. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Kruwinnus, M.; Weiss, J. Impact of different pan-frying conditions on the formation of heterocyclic aromatic amines and sensory quality in fried bacon. Food Chem. 2015, 168, 383–389. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, S.; Tao, G.J.; You, F.H.; Chen, J.; Zeng, M.M. Acetonitrile extraction coupled with UHPLC-MS/MS for the accurate quantification of 17 heterocyclic aromatic amines in meat products. J. Chromatogr. B 2017, 1068–1069, 173–179. [Google Scholar] [CrossRef]
- Özdestan, Ö.; Kaçar, E.; Keşkekoğlu, H.; Üren, Ali. Development of a new extraction method for heterocyclic aromatic amines determination in cooked meatballs. Food Anal. Methods 2014, 7, 116–126. [Google Scholar] [CrossRef]
- Yao, Y.; Peng, Z.Q.; Shao, B.; Wan, K.H.; Wang, F.L.; Zhang, Y.W.; Li, J.K.; Hui, T. Effects of frying and boiling on the formation of heterocyclic amines in braised chicken. Poultry Sci. 2013, 92, 3017–3025. [Google Scholar] [CrossRef]
- Lan, C.M.; Chen, B.H. Effects of soy sauce and sugar on the formation of heterocyclic amines in marinated foods. Food Chem. Toxicol. 2002, 40, 989–1000. [Google Scholar] [CrossRef]



| Analytes | Linear Range (μg/g) | R | Recovery ± RSD (%, n = 6) | Inter-Day RSD (%) | LOQs (ng/g) | LODs (ng/g) | ||
|---|---|---|---|---|---|---|---|---|
| Low (100 ng/g) | Middle (300 ng/g) | High (500 ng/g) | ||||||
| IQ | 0.012–12.0 | 0.9989 | 82.8 ± 4.6 | 82.1 ± 3.2 | 82.3 ± 3.1 | 3.9 | 4.2 | 3.0 |
| IQ [4,5-b] | 0.012–12.0 | 0.9994 | 78.6 ± 2.9 | 78.8 ± 4.4 | 78.5 ± 2.5 | 4.6 | 12.0 | 4.2 |
| Harman | 0.012–12.0 | 0.9986 | 90.9 ± 3.8 | 89.6 ± 3.5 | 89.6 ± 3.3 | 4.1 | 4.2 | 3.0 |
| Norharman | 0.012–12.0 | 0.9991 | 89.1 ± 3.5 | 88.1 ± 3.4 | 88.2 ± 3.3 | 4.4 | 4.2 | 3.0 |
| Phe-P-1 | 0.012–12.0 | 0.9983 | 103.2 ± 2.8 | 94.9 ± 0.6 | 101.6 ± 4.4 | 3.5 | 12.0 | 4.2 |
| Instrument | Extraction Method | Food Samples | Recovery (%) | RSD (%) | LOD | Sample Pretreatment Time (min) | Amounts of Adsorbent (mg/6 mL) | Ref. |
|---|---|---|---|---|---|---|---|---|
| UHPLC–APCI–MS/MS | SALLME 1 extraction/SPE purification | Cooked beef | 60.7–108.5 | <25.1 | 1–5 pg/µL | >100 | - | [11] |
| LC–MS-MS | QuEChERS | Meat | 58.9–117.4 | <25.6 | 0.003–0.05 ng/mL | 32 | 300 mg PSA + 900 mg MgSO4 + 300 mg C18EC | [12] |
| HPLC-DAD | SPE 2 | Chicken/beef | 43.0–92.049.0–98.0 | - | 0.3529–1.549 ng/g | >180 | - | [34] |
| UPLC–MS/MS | Extrelut-SPE | Roasted beef patties | 56.2–106.1 | <11.2 | 0.008–0.053 ng/g | 68.6 | - | [35] |
| UHPSFC–MS/MS | SPE | Grilled meals/fish | 52.3–97.5 | <6.0 | 0.01–0.05 µg/kg | - | - | [36] |
| UHPLC–MS/MS | Acetonitrile/SPE | Meat products | 42.5–99 | <12.2 | 0.007–0.202 ng/g | >50 | - | [37] |
| HPLC-DAD | SPE | Cooked meatballs | 68.9–87.8 | - | 0.04–1.40 ng/g | >60 | - | [38] |
| HPLC-DAD | Modified QuEChERS | Braised sauce beef | 78.5–103.2 | <4.6 | 3.0–4.2 ng/g | 4 | 35 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, P.; Zhang, X.; Wang, H.; Li, K.; Bai, Y. Development of a Modified QuEChERS Method Based on Magnetic Multi-Walled Carbon Nanotubes as a Clean-Up Adsorbent for the Analysis of Heterocyclic Aromatic Amines in Braised Sauce Beef. Foods 2023, 12, 138. https://doi.org/10.3390/foods12010138
Li M, Wang P, Zhang X, Wang H, Li K, Bai Y. Development of a Modified QuEChERS Method Based on Magnetic Multi-Walled Carbon Nanotubes as a Clean-Up Adsorbent for the Analysis of Heterocyclic Aromatic Amines in Braised Sauce Beef. Foods. 2023; 12(1):138. https://doi.org/10.3390/foods12010138
Chicago/Turabian StyleLi, Min, Pengxiang Wang, Xu Zhang, Hongyu Wang, Ke Li, and Yanhong Bai. 2023. "Development of a Modified QuEChERS Method Based on Magnetic Multi-Walled Carbon Nanotubes as a Clean-Up Adsorbent for the Analysis of Heterocyclic Aromatic Amines in Braised Sauce Beef" Foods 12, no. 1: 138. https://doi.org/10.3390/foods12010138