Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum frutescens L.) during Natural Fermentation
Abstract
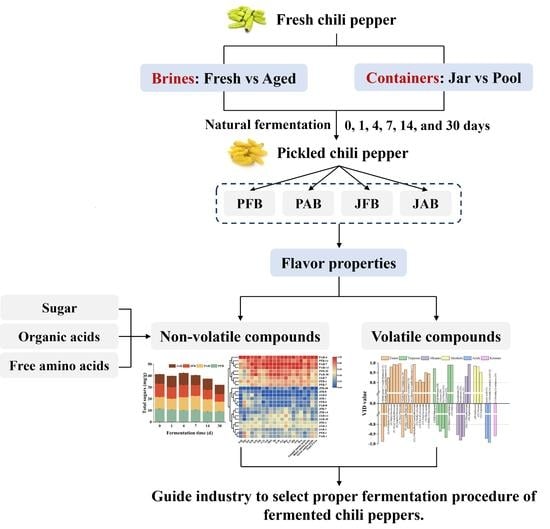
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation and Collection
2.3. pH Value
2.4. Organic Acids
2.5. Sugars
2.6. Free Amino Acids
2.7. Volatile Compounds
2.8. Data Analysis
2.8.1. Statistical Analysis
2.8.2. Multivariate Data Analysis (MVDA)
2.8.3. Kinetic Modeling
3. Results and Discussion
3.1. Taste Properties
3.1.1. pH Value
3.1.2. Organic Acids Profile
3.1.3. Sugars Profile
3.1.4. Free Amino Acids Contents
3.2. Aroma Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Bao, X.; Zhang, X.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Exploring the phytochemicals and inhibitory effects against α-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: Insights into mechanisms by molecular docking analysis. LWT 2022, 162, 113467. [Google Scholar] [CrossRef]
- Shang, Z.; Li, M.; Zhang, W.W.; Cai, S.; Hu, X.; Yi, J. Analysis of phenolic compounds in pickled chayote and their effects on antioxidant activities and cell protection. Food Res. Int. 2022, 157, 111325. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Shang, Z.; Li, M.; Qu, Y.; Long, H.; Yi, J. Evaluation of the physiochemical and aromatic qualities of pickled Chinese pepper (Paojiao) and their influence on consumer acceptability by using targeted and untargeted multivariate approaches. Food Res. Int. 2020, 137, 109535. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Shang, Z.; Zhang, S.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Dynamic analysis of flavor properties and microbial communities in Chinese pickled chili pepper (Capsicum Frutescens L.): A typical industrial-scale natural fermentation process. Food Res. Int. 2022, 153, 110952. [Google Scholar] [CrossRef]
- Shang, Z.; Ye, Z.; Li, M.; Ren, H.; Cai, S.; Hu, X.; Yi, J. Dynamics of microbial communities, flavor, and physicochemical properties of pickled chayote during an industrial-scale natural fermentation: Correlation between microorganisms and metabolites. Food Chem. 2022, 377, 132004. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Gong, C.; Tan, X.; Ren, Y.; Yao, K.; Chi, Y.; Zhang, Q. Physicochemical, microbial, and aroma characteristics of Chinese pickled red peppers (Capsicum Annuum) with and without biofilm. RSC Adv. 2020, 10, 6609–6617. [Google Scholar] [CrossRef] [Green Version]
- Rao, Y.; Tao, Y.; Chen, X.; She, X.; Qian, Y.; Li, Y.; Du, Y.; Xiang, W.; Li, H.; Liu, L. The characteristics and correlation of the microbial communities and flavors in traditionally pickled radishes. LWT 2020, 118, 108804. [Google Scholar] [CrossRef]
- Liu, L.; She, X.; Qian, Y.; Li, Y.; Tao, Y.; Che, Z.; Liu, G.; Rao, Y. Effect of different fermenting containers on the deterioration of Sichuan pickle. LWT 2019, 111, 829–836. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Liu, D. Biochemical changes and microbial community dynamics during spontaneous fermentation of Zhacai, a traditional pickled mustard tuber from China. Int. J. Food Microbiol. 2021, 347, 109199. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, S.; Zhang, X.; Jiang, Y.; Liu, Z.; Hu, X. Effects of pasteurization technologies and storage conditions on the flavor changes in acidified chili pepper. Curr. Res. Food Sci. 2022, 5, 1295–1304. [Google Scholar] [CrossRef]
- Ye, Z.; Shang, Z.; Li, M.; Zhang, X.; Ren, H.; Hu, X.; Yi, J. Effect of ripening and variety on the physiochemical quality and flavor of fermented Chinese chili pepper (Paojiao). Food Chem. 2022, 368, 130797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Lai, H.; Wang, Y.; Huang, Y.; Shi, Q.; He, W.; Zhu, S.; Li, Y.; Zhu, Y.; Li, H.; et al. Assessment of biogenic amine and nitrite production in low-salt Paocai during fermentation as affected by reused brine and fresh brine. Food Biosci. 2021, 41, 100958. [Google Scholar] [CrossRef]
- Liu, L.; She, X.; Chen, X.; Qian, Y.; Tao, Y.; Li, Y.; Guo, S.; Xiang, W.; Liu, G.; Rao, Y. Microbiota succession and chemical composition involved in the radish fermentation process in different containers. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Wei, B.; Huang, T.; Xiao, Y.; Peng, Z.; Xie, M.; Xiong, T. Bacterial community and composition in Jiang-shui and Suan-cai revealed by high-throughput sequencing of 16S rRNA. Int. J. Food Microbiol. 2019, 306, 108271. [Google Scholar] [CrossRef] [PubMed]
- Stoll, D.A.; Müller, A.; Meinhardt, A.K.; Dötsch, A.; Greiner, R.; Kulling, S.E.; Huch, M. Influence of salt concentration and iodized table salt on the microbiota of fermented cucumbers. Food Microbiol. 2020, 92, 103552. [Google Scholar] [CrossRef]
- Kurihara, H.; Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 2004, 274, 161–169. [Google Scholar] [CrossRef]
- Tola, Y.B.; Ramaswamy, H.S. Evaluation of high pressure (HP) treatment for rapid and uniform pH reduction in carrots. J. Food Eng. 2013, 116, 900–909. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Hou, Q.; Xu, H.; Zheng, Y.; Zhang, H.; Zhang, L. Assessment of bacterial profiles in aged, home-made Sichuan paocai brine with varying titratable acidity by PacBio SMRT sequencing technology. Food Control 2017, 78, 14–23. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef]
- Satora, P.; Skotniczny, M.; Strnad, S.; Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 2021, 136, 110325. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, B.; Lu, W.; Liu, X.; Zhao, J.; Ge, L.; Zhu, Y.; Lai, H.; Paul Ross, R.; Chen, W.; et al. Divergent role of abiotic factors in shaping microbial community assembly of paocai brine during aging process. Food Res. Int. 2020, 137, 109559. [Google Scholar] [CrossRef] [PubMed]
- D’Este, M.; Alvarado-Morales, M.; Angelidaki, I. Amino acids production focusing on fermentation technologies–A review. Biotechnol. Adv. 2018, 36, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Z.; Lu, J.R. Strategies for Enhancing Fermentative Production of Acetoin: A Review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.M.; Beresford, T.P.; Steele, J.; Broadbent, J.; Shah, N.P.; Ustunol, Z. Invited review: Advances in starter cultures and cultured Foods. J. Dairy Sci. 2007, 90, 4005–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Luo, W.; Peng, Y.; Niu, K.; Liu, X.; Shen, G.; Zhang, Z.; Wan, H.; Luo, Q.; Li, S. Quality and microbial flora changes of radish paocai during multiple fermentation rounds. Food Control 2019, 106, 106733. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, X.; Xie, S.; Liu, Y.; Zhang, M.; Che, Z.; Liu, Y.; Liu, P.; Lin, H. Dynamics and correlation of microbial community and flavor in Pixian Douban fermented with closed process of constant temperature. J. Sci. Food Agric. 2021, 101, 4142–4153. [Google Scholar] [CrossRef]
- Rao, Y.; Qian, Y.; Tao, Y.; She, X.; Li, Y.; Chen, X.; Guo, S.; Xiang, W.; Liu, L.; Du, H.; et al. Characterization of the microbial communities and their correlations with chemical profiles in assorted vegetable Sichuan pickles. Food Control 2020, 113, 107174. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, F.; Shi, X.; Wang, B.; Li, K.; Li, B.; Zhuge, B. Dynamic correlations between microbiota succession and flavor development involved in the ripening of Kazak artisanal cheese. Food Res. Int. 2018, 105, 733–742. [Google Scholar] [CrossRef]
- Del Rio, B.; Alvarez-Sieiro, P.; Redruello, B.; Martin, M.C.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Lactobacillus rossiae strain isolated from sourdough produces putrescine from arginine article. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, J.S.; Seeras, A.; Sanchez-Maldonado, A.F.; Zhang, C.; Su, M.S.W.; Gänzle, M.G. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiol. 2014, 42, 172–180. [Google Scholar] [CrossRef]
- Suharta, S.; Hunaefi, D.; Wijaya, C.H. Changes in volatiles and aroma profile of andaliman (Zanthoxylum acanthopodium DC.) upon various drying techniques. Food Chem. 2021, 365, 130483. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; He, P.; Yang, Y.; Qiao, Z.; Huang, D.; Luo, H.; Feng, X. Occurrence, diversity, and character of Bacillaceae in the solid fermentation process of strong aromatic liquors. Front. Microbiol. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tang, H.; Ou, C.; Xie, C.; Cao, J.; Zhang, X. A comparative study of volatile flavor components in four types of zaoyu using comprehensive two-dimensional gas chromatography in combination with time-of-flight mass spectrometry. J. Food Process. Preserv. 2021, 45, 1–27. [Google Scholar] [CrossRef]
- Tufariello, M.; Durante, M.; Ramires, F.A.; Grieco, F.; Tommasi, L.; Perbellini, E.; Falco, V.; Tasioula-margari, M. New process for production of fermented black table olives using selected autochthonous microbial resources. Front. Microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, M.; Akgül, A. Storage quality in different brines of pickled capers (Capparis Spp.). Grasas Aceites 1999, 50, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cao, J.; Xu, H.; Hou, Q.; Yu, Z.; Zhang, H.; Sun, Z. Bacterial diversity and community structure in Chongqing radish paocai brines revealed using PacBio single-molecule real-time sequencing technology. J. Sci. Food Agric. 2018, 98, 3234–3245. [Google Scholar] [CrossRef]
- González-Marco, A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Concentration of volatile compounds in Chardonnay wine fermented in stainless steel tanks and oak barrels. Food Chem. 2008, 108, 213–219. [Google Scholar] [CrossRef]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by HS–SPME with GC–MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef]
- Mozūraitis, R.; Apšegaitė, V.; Radžiutė, S.; Aleknavičius, D.; Būdienė, J.; Stanevičienė, R.; Blažytė-Čereškienė, L.; Servienė, E.; Būda, V. Volatiles produced by yeasts related to Prunus avium and P. cerasus fruits and their potentials to modulate the behaviour of the pest Rhagoletis cerasi fruit flies. J. Fungi 2022, 8, 95. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of yeasts on the sensory component of wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Shekhawat, K.; Porter, T.J.; Bauer, F.F.; Setati, M.E. Employing Oxygen Pulses to Modulate Lachancea Thermotolerans–Saccharomyces Cerevisiae Chardonnay Fermentations. Ann. Microbiol. 2018, 68, 93–102. [Google Scholar] [CrossRef]




 ), pool with aged brine (
), pool with aged brine ( ), jar with fresh brine (
), jar with fresh brine ( ), and jar with aged brine (
), and jar with aged brine ( ) for pickled chili peppers of different fermentation procedures (n = 3). The X− and Y−variance explained by LV1 and LV2 (A), LV1 and LV3 (B), LV2 and LV3 (C) were indicated in the respective axes. The open circles show different volatiles. The discriminant volatiles with absolute VID values above 0.800 were named and marked in bold.
) for pickled chili peppers of different fermentation procedures (n = 3). The X− and Y−variance explained by LV1 and LV2 (A), LV1 and LV3 (B), LV2 and LV3 (C) were indicated in the respective axes. The open circles show different volatiles. The discriminant volatiles with absolute VID values above 0.800 were named and marked in bold.
 ), pool with aged brine (
), pool with aged brine ( ), jar with fresh brine (
), jar with fresh brine ( ), and jar with aged brine (
), and jar with aged brine ( ) for pickled chili peppers of different fermentation procedures (n = 3). The X− and Y−variance explained by LV1 and LV2 (A), LV1 and LV3 (B), LV2 and LV3 (C) were indicated in the respective axes. The open circles show different volatiles. The discriminant volatiles with absolute VID values above 0.800 were named and marked in bold.
) for pickled chili peppers of different fermentation procedures (n = 3). The X− and Y−variance explained by LV1 and LV2 (A), LV1 and LV3 (B), LV2 and LV3 (C) were indicated in the respective axes. The open circles show different volatiles. The discriminant volatiles with absolute VID values above 0.800 were named and marked in bold.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Xiao, Y.; Jiang, Y.; Wang, T.; Cai, S.; Hu, X.; Yi, J. Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum frutescens L.) during Natural Fermentation. Foods 2023, 12, 101. https://doi.org/10.3390/foods12010101
Zhang S, Xiao Y, Jiang Y, Wang T, Cai S, Hu X, Yi J. Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum frutescens L.) during Natural Fermentation. Foods. 2023; 12(1):101. https://doi.org/10.3390/foods12010101
Chicago/Turabian StyleZhang, Shiyao, Yue Xiao, Yongli Jiang, Tao Wang, Shengbao Cai, Xiaosong Hu, and Junjie Yi. 2023. "Effects of Brines and Containers on Flavor Production of Chinese Pickled Chili Pepper (Capsicum frutescens L.) during Natural Fermentation" Foods 12, no. 1: 101. https://doi.org/10.3390/foods12010101