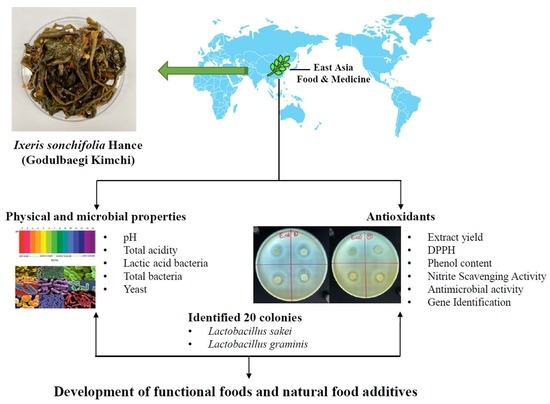
Effect of Fermentation Duration on the Quality Changes of Godulbaegi Kimchi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Preparation of Godulbaegi Kimchi
2.2. Sampling, pH, and Total Acidity
2.3. Number of LAB, Total Bacteria, and Yeast
2.4. Sample Preparation and Extraction Yield
2.5. Determination of Total Polyphenols
2.6. Measurement of Free-Radical-Scavenging Activity
2.7. Nitrite Scavenging Activity
- A.
- The absorbance of 1 mM NaNO2 added sample after allowing to stand for 1 h;
- B.
- The absorbance of control;
- C.
- The absorbance of 1 mM NaNO2.
2.8. Antimicrobial Activity
2.9. Identification of LAB from Godulbaegi Kimchi
2.10. Statistical Analysis
3. Results and Discussion
3.1. pH and Acidity Value of Godulbaegi Kimchi
3.2. Microbial Load Analysis
3.3. Extraction Yield
3.4. Total Phenolic Content
3.5. DPPH Radical Scavenging
3.6. Nitrite Scavenging in Godulbaegi Kimchi
3.7. Antimicrobial Activity of Lactic Acid Bacteria Isolates against Target Bacteria
3.8. Identification by 16S rRNA Sequencing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, W.; Zhang, J.Y.; Dong, L.Y.; Yin, P.H.; Wang, C.G.; Lu, J.Q.; Zhang, H.G. Identification of the metabolites of Ixerin Z from Ixeris sonchifolia Hance in rats by HPLC–LTQ-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2015, 107, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Bog, Y.S.; Hwa, L.J.; Young, C.H.; Sik, I.K.; Ja, B.S.; Soo, C.J.; Deuk, K.N. Inhibitory effects of luteolin isolated from Ixeris sonchifolia Hance on the proliferation of HepG2 human hepatocellular carcinoma cells. Arch. Pharm. Res. 2003, 26, 151–156. [Google Scholar] [CrossRef]
- Chen, C.G.; Jia, H.L.; Lv, S.X.; Xu, C.Q. Protective effect of Kudiezi on acute cerebral ischemic reperfusion injury in rats. Chin. J. Clin. Pharmacol. 2012, 28, 196–199. [Google Scholar]
- Cendrowski, A.; Kraśniewska, K.; Przybył, J.L.; Zielińska, A.; Kalisz, S. Antibacterial and Antioxidant Activity of Extracts from Rose Fruits (Rosa rugosa). Molecules 2020, 25, 1365. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.Y.; Yu, X.F.; Qu, S.C.; Xu, H.L.; Sui, D.Y. Protective effects of total flavonoids of sowthistle-leaf ixeris sedling on myocardial ischemia reperfusion injury in rats. Chin. Pharmacol. B 2010, 2, 276–277. [Google Scholar]
- Zhai, R.Y.; Huang, X.N.; Yu, X.F.; Qu, S.C.; Sui, D.Y. Effect of Ixeris sonchifolia extracts (KDZ) for injection on myocardial enzymes and blood viscosities of myocardial ischemia reperfusion injury rats. Pharmacol. Clin. Chin. Mater. Med. 2008, 24, 53–55. [Google Scholar]
- Ryu, H.S.; Kim, J.H.; Kim, H.S. Effects of a plant water extract mixture (Ixeris sonchifolia Hance, Oenanthe javanica, Fagopyrum esculentum Moench, Hizikia fusiforme, Zingiber officinale Roscoe) on mouse immune cell activation. Korean J. Food Nutr. 2007, 20, 74–78. [Google Scholar] [CrossRef]
- Li, K.W.; Liang, Y.Y.; Xie, S.M.; Niu, F.J.; Guo, L.Y.; Liu, Z.H.; Zhou, C.Z.; Wang, L.Z. Ixeris sonchifolia: A review of its traditional uses, chemical constituents, pharmacology and modern applications. Biomed. Pharmacother. 2020, 125, 109869. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C. Comparison of the properties of Youngia sonchifolia Max. for Kimchi preparation. Korean J. Plant Res. 1996, 9, 105–112. [Google Scholar]
- Wang, X.; Li, Y.; Chen, M.; Li, C.; Huang, W.; Gu, K.; Yu, H.; Yuan, Y.; Wang, Y.; Yang, B.; et al. Stepwise rapid tracking strategy to identify active molecules from Ixeris sonchifolia Hance based on “‘affinity mass spectrometry-atomic force microscopy imaging’” technology. Talanta 2020, 217, 121031. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Feng, X.; Sun, Q.; Lu, H.; Manabe, M.; Sugahara, K.; Ma, D.; Sagara, Y.; Kodama, H. Effect of six flavonoid compounds from Ixeris sonchifolia on stimulus-induced superoxide generation and tyrosyl phosphorylation in human neutrophils. Clin. Chim. Acta 2002, 316, 95–99. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Ryu, H.S.; Chun, S.S.; Park, K.Y.; Rhee, S.H. Effect of godulbaegi (Korean lettuce, Ixeris sonchifolia H.) kimchi on the in vitro digestibility of proteins. J. Korean Soc. Food Nutr. 1995, 24, 1010–1015. [Google Scholar]
- Yang, J.Y.; Yu, J.L.; Dai, Z.; Liu, H.S.; Bai, W. Regulation effect of Kudiezi Injection on PKCδ/MARCKS signal pathway in vascular endothelial cells after hypoxia injury. J. Beijing Univ. Tradit. Chin. Med. 2017, 40, 214–218. [Google Scholar]
- Liu, X.M.; Zhang, X.Y.; Wang, F.L.; Liang, X.; Zeng, Z.X.; Zhao, J.Y.; Zheng, H.; Jiang, X.N.; Zhang, Y.L. Improvement in cerebral ischemia–reperfusion injury through the TLR4/NFκB pathway after Kudiezi injection in rats. Life Sci. 2017, 191, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Tao, Y.; Wang, F.L.; Yao, T.; Fu, C.; Zheng, H.; Yan, Y.; Liang, X.; Jiang, X.N.; Zhang, Y.L. Kudiezi injection mitigates myocardial injury induced by acute cerebral ischemia in rats. BMC Complement. Altern. Med. 2017, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.Y.; Wu, Y.Y.; Yuan, L.; Wang, Y.; Tian, M.; Li, Y.B.; Yang, B. Analyze difference of chemical compositions in Ixeris sonchifolia from different origins by UPLC-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 37–42. [Google Scholar]
- Feng, X.Z.; Dong, M.; Gao, Z.J.; Xu, S.X. Three new triterpenoid saponins from Ixeris sonchifolia and their cytotoxic activity. Planta Med. 2003, 69, 1036–1040. [Google Scholar] [PubMed]
- Kim, H.R.; Lee, I.S. Quality changes of godulbaegi (Youngia sonchifolia Maxim) kimchi during storage at different temperatures. Korean J. Food Cookery Sci. 2008, 24, 617–625. [Google Scholar]
- Kim, J.M.; Kim, J.N.; Lee, K.S.; Shin, J.R.; Yoon, K.Y. Comparison of physicochemical properties of wild and cultivated Lactuca indica. J. Korean Soc. Food Sci. Nutr. 2012, 41, 526–532. [Google Scholar] [CrossRef]
- Lee, J.J.; Choi, Y.J.; Lee, M.J.; Park, S.J.; Oh, S.J.; Yun, Y.R.; Min, S.G.; Seo, H.Y.; Park, S.H.; Lee, M.A. Effects of combining two lactic acid bacteria as a starter culture on model kimchi fermentation. Food Res. Int. 2020, 136, 109591. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F.; Hamed, I. The importance of lactic acid bacteria for the prevention of bacterial growth and their biogenic amines formation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety, 2022. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=374 (accessed on 20 February 2022).
- Ministry of Food and Drug Safety, 2022. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=362 (accessed on 20 February 2022).
- Ministry of Food and Drug Safety, 2022. Available online: https://www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=377 (accessed on 20 February 2022).
- Mohd-Esa, N.; Hern, F.S.; Ismail, A.; Yee, C.L. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010, 122, 1055–1060. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Gray, J.I.; Dugan Jr, L.R. Inhibition of n-nitrosamine formation in model food systems. J. Food Sci. 1975, 40, 981–984. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yong, S.; Lee, M.J.; Park, S.J.; Yun, Y.R.; Park, S.H.; Lee, M.A. Changes in volatile and non-volatile compounds of model kimchi through fermentation by lactic acid bacteria. LWT-Food Sci. Technol. 2019, 105, 118–126. [Google Scholar] [CrossRef]
- Park, Y.H.; Seo, H.J.; Cho, I.Y.; Han, J.G.; Chun, H.K. Changes of quality characteristics and nitrate contents in ulgari-baechu kimchi, yulmoo kimchi and yulmoo mul-kimchi during storage period. J. Food Sci. Nutr. 2007, 36, 794–799. [Google Scholar]
- Jung, S.J.; Jang, M.S. Effect of wasabi (Wasabia japonica matsum) stalk on the fermentation of baechukimchi. Food Sci. Biotechnol. 2008, 17, 692–699. [Google Scholar]
- Baek, S.H.; Maruthupandy, M.; Lee, K.E.; Kim, D.W.; Seo, J.C. Freshness indicator for monitoring changes in quality of packaged kimchi during storage. Food Packag. Shelf Life 2020, 25, 100528. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, J.H.; Min, S.; Min, D.B. Changes of volatile compounds, lactic acid bacteria, pH, and headspace gases in kimchi, a traditional Korean fermented vegetable product. J. Food Sci. 2003, 68, 849–854. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, M.J.; Lim, J.Y.; Hong, J.H. Cross-cultural comparison of consumer acceptability of kimchi with different degree of fermentation. J. Sens. Stud. 2016, 31, 124–134. [Google Scholar] [CrossRef]
- Saithong, P.; Panthavee, W.; Boonyaratanakornkit, M.; Sikkhamondhol, C. Use of a starter culture of lactic acid bacteria in plaa-som, a Thai fermented fish. J. Biosci. Bioeng. 2010, 110, 553–557. [Google Scholar] [CrossRef]
- Lim, S.M.; Lee, N.K.; Kim, K.T.; Paik, H.D. Probiotic Lactobacillus fermentum KU200060 isolated from watery kimchi and its application in probiotic yogurt for oral health. Microb. Pathog. 2020, 147, 104430. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Franco, D.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Lema, J.M. Evaluation of extracts from Gevuina avellana hulls as antioxidants. J. Agric. Food Chem. 2000, 48, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Jeon, Y.S.; Han, J.S. Antioxidative activity of mustard leaf kimchi added green tea and pumpkin powder. J. Korean Soc. Food Sci. Nutr. 2001, 30, 1053–1059. [Google Scholar]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT-Food Sci. Technol. 2017, 86, 438–446. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.H.; Sung, N.J. Inhibitory effect of whole strawberries, garlic juice or kale juice on endogenous formation of N-nitrosodimethylamine in humans. Cancer Lett. 2002, 182, 1–10. [Google Scholar] [CrossRef]
- Lee, G.C.; Kim, Y.K. Changes in fermentation characteristics of kimchi added with leek. J. Korean Soc. Food Sci. Nutr. 1999, 28, 780–785. [Google Scholar]
- Ammor, S.; Dufour, E.; Zagorec, M.; Chaillou, S.; Chevallier, I. Characterization and selection of Lactobacillus sakei strains isolated from traditional dry sausage for their potential use as starter cultures. Food Microbiol. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Park, J.M.; Shin, J.H.; Gu, J.G.; Yoon, S.J.; Song, J.C.; Jeon, W.M.; Suh, H.J.; Chang, U.J.; Yang, C.Y.; Kim, J.M. Effect of antioxidant activity in kimchi during a short-term and over-ripening fermentation period. J. Biosci. Bioeng. 2011, 112, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.H.; Qi, T.; Hong, Y.; Deng, L.L.; Zeng, K.F. Screening of bacteriocin-producing lactic acid bacteria in Chinese homemade pickle and dry-cured meat, and bacteriocin identification by genome sequencing. LWT-Food Sci. Technol. 2020, 125, 109177. [Google Scholar] [CrossRef]
- Champomier-Vergès, M.C.; Chaillou, S.; Cornet, M.; Zagorec, M. Lactobacillus sakei: Recent developments and future prospects. Res. Microbiol. 2001, 152, 839–848. [Google Scholar] [CrossRef]
- Lee, H.J.; Yoon, H.S.; Ji, Y.S.; Kim, H.N.; Park, H.J.; Lee, J.E.; Shin, H.K.; Holzapfel, W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011, 145, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Çakır, E.; Arıcı, M.; Durak, M.Z. Biodiversity and techno-functional properties of lactic acid bacteria in fermented hull-less barley sourdough. J. Biosci. Bioeng. 2020, 130, 450–456. [Google Scholar] [CrossRef]


| SGK a | LGK b | |
|---|---|---|
| pH | 4.43 ± 0.14 c | 4.20 ± 0.13 |
| Total acidity (%) | 0.46 ± 0.02 | 0.92 ± 0.03 |
| Lactic acid bacteria (CFU/mL) | 6.06 ± 0.28 | 6.59 ± 0.10 |
| Total viable count (CFU/mL) | 5.85 ± 0.34 | 4.66 ± 0.13 |
| Total yeast count (CFU/mL) | 1.24 ± 0.14 | 3.06 ± 0.03 |
| Extract Solvent | Yield Extraction (%) | Total Phenolic Content (µg of GAEs/mg Extract) |
|---|---|---|
| LGK 1 | ||
| Ethanol | 52.58 ± 2.06 b | 77.40 ± 1.42 a |
| Methanol | 61.51 ± 1.21 a | 79.01 ± 2.36 a |
| Water | 48.54 ± 0.97 c | 38.02 ± 0.80 b |
| Concentration (%) | pH | Ethanol | Methanol | Water |
|---|---|---|---|---|
| LGK; Long-term fermented Godulbaegi kimchi | 1.2 | 80.97 ± 0.87 aA | 81.90 ± 2.17 aA | 74.86 ± 2.05 bA |
| 3.0 | 72.30 ± 1.96 aB | 72.80 ± 1.50 aB | 54.44 ± 2.03 bB | |
| 6.0 | 26.70 ± 0.96 aC | 30.49 ± 2.04 aC | 21.32 ± 1.25 bC |
| Strain No.a | Negative Control b | Escherichia coli | Salmonella typhimurium | Staphylococcus aureus | Listeria monocytogenes |
|---|---|---|---|---|---|
| 1 | - c | +++ | ++ | - | - |
| 2 | - | ++ | - | - | - |
| 3 | - | ++ | - | - | - |
| 4 | - | ++ | ++ | - | - |
| 5 | - | ++ | - | - | - |
| 6 | - | ++ | ++ | - | - |
| 7 | - | ++ | - | - | - |
| 8 | - | ++ | - | - | - |
| 9 | - | ++ | ++ | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Zhang, B.-Z.; Kim, J.-M. Effect of Fermentation Duration on the Quality Changes of Godulbaegi Kimchi. Foods 2022, 11, 1020. https://doi.org/10.3390/foods11071020
Park J-M, Zhang B-Z, Kim J-M. Effect of Fermentation Duration on the Quality Changes of Godulbaegi Kimchi. Foods. 2022; 11(7):1020. https://doi.org/10.3390/foods11071020
Chicago/Turabian StylePark, Jung-Min, Bo-Zheng Zhang, and Jin-Man Kim. 2022. "Effect of Fermentation Duration on the Quality Changes of Godulbaegi Kimchi" Foods 11, no. 7: 1020. https://doi.org/10.3390/foods11071020