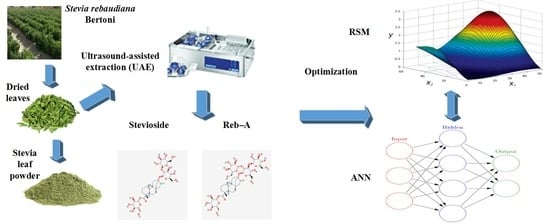
A Hybrid RSM-ANN-GA Approach on Optimization of Ultrasound-Assisted Extraction Conditions for Bioactive Component-Rich Stevia rebaudiana (Bertoni) Leaves Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stevia Leaf Powder Preparation, Reagents and Chemicals
2.2. Conventional Maceration Extraction Procedure
2.3. Ultrasound Assisted Extraction Procedure
2.4. Preliminary Screening Study and RSM-Based Experimental Design
2.5. Artificial Neural Network (ANN) Modeling
2.6. Genetic Algorithm
2.7. Determination of Total Extract Yield (TEY)
2.8. HPLC Analysis
2.9. Statistical Analysis
Performance Comparison of RSM and ANN-GA Models
3. Results and Discussion
3.1. RSM Modeling of UAE Process
3.2. Process Variables Effect on Total Extract Yield (YEY)
3.3. Process Variables Effect on ST Yield
3.4. Process Variables Effect on Reb-A Yield
3.5. Hybrid ANN–GA Modeling
3.6. Predicive Performance Comparison of RSM and ANN–GA Models
3.7. PCA
3.8. Physicochemical Features and Glycosides Extraction Phenomenon
3.9. Comparison of Extraction Efficiencies of UAE and CME
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ameer, K.; Bae, S.-W.; Jo, Y.; Lee, H.-G.; Ameer, A.; Kwon, J.-H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef]
- Ahmad, J.; Khan, I.; Blundell, R.; Azzopardi, J.; Mahomoodally, M.F. Stevia rebaudiana Bertoni.: An updated review of its health benefits, industrial applications and safety. Trends Food Sci. Technol. 2020, 100, 177–189. [Google Scholar] [CrossRef]
- Movahedian, M.; Golzan, S.A.; Ashtary-Larky, D.; Clark, C.C.; Asbaghi, O.; Hekmatdoost, A. The effects of artificial-and stevia-based sweeteners on lipid profile in adults: A Grade-assessed systematic review, meta-analysis, and meta-regression of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Chun, B.-S.; Kwon, J.-H. Optimization of supercritical fluid extraction of steviol glycosides and total phenolic content from Stevia rebaudiana (Bertoni) leaves using response surface methodology and artificial neural network modeling. Ind. Crops Prod. 2017, 109, 672–685. [Google Scholar] [CrossRef]
- Edwards, C.H.; Rossi, M.; Corpe, C.P.; Butterworth, P.J.; Ellis, P.R. The role of sugars and sweeteners in food, diet and health: Alternatives for the future. Trends Food Sci. Technol. 2016, 56, 158–166. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Ultrasonic vs. microwave extraction intensification of active principles from medicinal plants. Proc. AIDIC Conf. Ser. 2009, 1, 1–14. [Google Scholar]
- Jaitak, V.; Kaul, V.K.; Kumar, N.; Singh, B.; Savergave, L.; Jogdand, V.; Nene, S. Simple and efficient enzymatic transglycosylation of stevioside by β-cyclodextrin glucanotransferase from Bacillus firmus. Biotechnol. Lett. 2009, 31, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Dacome, A.S.; da Silva, C.C.; da Costa, C.E.M.; Fontana, J.D.; Adelmann, J.; da Costa, S.C. Sweet diterpenic glycosides balance of a new cultivar of Stevia rebaudiana (Bert.) Bertoni: Isolation and quantitative distribution by chromatographic, spectroscopic, and electrophoretic methods. Process Biochem. 2005, 40, 3587–3594. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. UPB Sci. Bull. B Chem. Mater. Sci. B 2012, 74, 1454–2331. [Google Scholar]
- Javad, S.; Naz, S.; Ilyas, S.; Tariq, A.; Aslam, F. Optimization of the microwave assisted extraction and its comparison with different conventional extraction methods for isolation of stevioside from Stevia rebaudiana. Asian J. Chem. 2014, 26, 8043. [Google Scholar] [CrossRef]
- Rai, C.; Majumdar, G.C.; De, S. Optimization of process parameters for water extraction of stevioside using response surface methodology. Sep. Sci. Technol. 2012, 47, 1014–1022. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.-W.; Tang, J. Ultrasonically assisted extraction of total carbohydrates from Stevia rebaudiana Bertoni and identification of extracts. Food Bioprod. Process. 2010, 88, 215–221. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-G.; Jo, Y.; Ameer, K.; Kwon, J.-H. Optimization of green extraction methods for cinnamic acid and cinnamaldehyde from cinnamon (Cinnamomum cassia) by response surface methodology. Food Sci. Biotechnol. 2018, 27, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Bae, S.-W.; Jo, Y.; Chung, N.; Gao, Y.; Kwon, J.-H. Optimization and modeling for heat reflux extraction of total yield, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves. Sep. Sci. Technol. 2017, 52, 1193–1205. [Google Scholar] [CrossRef]
- Yahya, H.S.M.; Abbas, T.; Amin, N.A.S. Optimization of hydrogen production via toluene steam reforming over Ni–Co supported modified-activated carbon using ANN coupled GA and RSM. Int. J. Hydrogen Energy 2020, 46, 24632–24651. [Google Scholar] [CrossRef]
- Rouhani, M. Modeling and optimization of ultrasound-assisted green extraction and rapid HPTLC analysis of stevioside from Stevia rebaudiana. Ind. Crop Prod. 2019, 132, 226–235. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Uygun, Ö.; Bircan, C. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques. Sep. Sci. Technol. 2021, 56, 936–948. [Google Scholar] [CrossRef]
- Pól, J.; Ostrá, E.V.; Karásek, P.; Roth, M.; Benešová, K.; Kotlaříková, P.; Čáslavský, J. Comparison of two different solvents employed for pressurised fluid extraction of stevioside from Stevia rebaudiana: Methanol versus water. Anal. Bioanal. Chem. 2007, 388, 1847–1857. [Google Scholar] [CrossRef]
- Martins, P.M.; Thorat, B.N.; Lanchote, A.D.; Freitas, L.A.P. Green extraction of glycosides from Stevia rebaudiana (Bert.) with low solvent consumption: A desirability approach. Resour. Effic. Technol. 2016, 2, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Periche, A.; Castelló, M.L.; Heredia, A.; Escriche, I. Influence of extraction methods on the yield of steviol glycosides and antioxidants in Stevia rebaudiana extracts. Plant Foods Hum. Nutr. 2015, 70, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Šic Žlabur, J.; Voca, S.; Dobricevic, N.; Brncic, M.; Dujmic, F.; Rimac Brnčić, S. Optimization of ultrasound assisted extraction of functional ingredients from Stevia rebaudiana Bertoni leaves. Int. Agrophys. 2015, 29, 231–237. [Google Scholar] [CrossRef]
- Yasemi, M.; Rahimi, M.; Heydarinasab, A.; Ardjmand, M. Optimization of microfluidic gallotannic acid extraction using artificial neural network and genetic algorithm. Chem. Prod. Process Model. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Kumar, H.; Kumar, V. Ultrasonication assisted formation and stability of water-in-oil nanoemulsions: Optimization and ternary diagram analysis. Ultrason. Sonochem. 2018, 49, 79–88. [Google Scholar] [CrossRef]
- Erkucuk, A.; Akgun, I.; Yesil-Celiktas, O. Supercritical CO2 extraction of glycosides from Stevia rebaudiana leaves: Identification and optimization. J. Supercrit. Fluid 2009, 51, 29–35. [Google Scholar] [CrossRef]
- JECFA/FAO. Steviol Glycosides. Steviol Glycosides. Evaluation of Certain Food Additives: 73rd Report of the Joint FAO/WHO Expert Committee on Food Additives. FAO/JECFA Monograph; JECFA/FAO: Rome, Italy, 2010; pp. 63–71. [Google Scholar]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.S.; Aboutabl, E.A.; Hammouda, F.M. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L. (artichoke) leaves. Ultrason. Sonochem. 2016, 31, 330–336. [Google Scholar] [CrossRef]
- Shao, P.; Jiang, S.; Ying, Y. Optimization of molecular distillation for recovery of tocopherol from rapeseed oil deodorizer distillate using response surface and artificial neural network models. Food Bioprod. Process. 2007, 85, 85–92. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind. Crops Prod. 2012, 40, 247–253. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, L.; Yang, Z.; Yao, S.; Song, H. The preparation of high purity of rebaudioside A and stevioside and the enrichment of rebaudioside C by subsequent twice crystallizations from natural stevia resources. Separations 2021, 8, 200. [Google Scholar] [CrossRef]
- Yen, N.T.H. Chemical composition of dried Bertoni leaves and effect of ultrasound-assisted extraction on total steviosides content in extract. Herba Pol. 2021, 67, 1–7. [Google Scholar] [CrossRef]
- Choi, H.J.; Naznin, M.; Alam, M.B.; Javed, A.; Alshammari, F.H.; Kim, S.; Lee, S.H. Optimization of the extraction conditions of Nypa fruticans Wurmb. using response surface methodology and artificial neural network. Food Chem. 2022, 381, 132086. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, J.L.; Preston, C.; Gomes, R.L. Comparison of response surface methodology (RSM) and artificial neural networks (ANN) towards efficient extraction of artemisinin from Artemisia annua. Ind. Crops Prod. 2014, 58, 15–24. [Google Scholar] [CrossRef]
- Teslić, N.; Bojanić, N.; Rakić, D.; Takači, A.; Zeković, Z.; Fišteš, A.; Pavlić, B. Defatted wheat germ as source of polyphenols—Optimization of microwave-assisted extraction by RSM and ANN approach. Chem. Eng. Process. Process. Intensif. 2019, 143, 107634. [Google Scholar] [CrossRef]
- Jaworska, K.; Krynitsky, A.J.; Rader, J.I. Simultaneous analysis of steviol and steviol glycosides by liquid chromatography with ultraviolet detection on a mixed-mode column: Application to stevia plant material and stevia-containing dietary supplements. J. AOAC Int. 2012, 95, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Ishizuki, K.; Iwamura, J.; Mikami, H.; Hirao, Y.; Fujita, I.; Yamazaki, T.; Akiyama, H.; Kawamura, Y. Improvement of the assay method for steviol glycosides in the JECFA specifications. Am. J. Analyt. Chem. 2013, 04, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, B.F.; Woelwer-Rieck, U.; Papagiannopoulos, M. Separation of steviol glycosides by hydrophilic liquid interaction chromatography. Food Anal. Methods 2012, 5, 266–271. [Google Scholar] [CrossRef]
- Arslan Kulcan, A.; Karhan, M. Effect of process parameters on stevioside and rebaudioside A content of stevia extract obtained by decanter centrifuge. J. Food Process. Preserv. 2021, 45, e15168. [Google Scholar] [CrossRef]
- Cravotto, G.; Binello, A.; Orio, L. Green extraction techniques. Agro Food Ind. Hi-Tech 2011, 22, 57–59. [Google Scholar]
- Heleno, S.A.; Diz, P.; Prieto, M.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- IPCC. IPCC Guidelines. Revised IPCC Guidelines for National Greenhouse Gas Inventories; Bracknell, UK, 1996; Available online: http://www.ipcc-nggip.iges.or.jp/public/gl/invs1.html (accessed on 3 March 2022).
- MTIE. Implementing Regulations in Energy Law; Ministry of Trade, Industry and Energy (MTIE): Sejong, Korea, 2011; Volume 5. [Google Scholar]
- KPX. Korea Power Exchange. Available online: https://new.kpx.or.kr/board.es?mid=a20505000000&bid=0057 (accessed on 21 February 2022).









| Input Variable | Variable Range and Levels (Coded) | ||||||
|---|---|---|---|---|---|---|---|
| Unit | Code | −1.68 (−α) | −1 | 0 | 1 | 1.68 (+α) | |
| Ethanol concentration | % | X1 | 0 | 25 | 50 | 75 | 100 |
| Sonication time | min | X2 | 10 | 21 | 32 | 43 | 54 |
| Leaf to solvent ratio | g/mL | X3 | 0.148 | 0.18 | 0.23 | 0.28 | 0.313 |
| Dependent Variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run No. 2 | Independent Variables 1 | Y1: TE Yield (%) | Y2: ST Yield (mg/g) | Y3: Reb-A Yield (mg/g) | ||||||||
| X1 (%) | X2 (min) | X3 (g/mL) | Experimental Data | RSM Predicted | ANN Predicted | Experimental Data | RSM Predicted | ANN Predicted | Experimental Data | RSM Predicted | ANN Predicted | |
| 1 | 25 (−1) | 21 (−1) | 0.18 (−1) | 5.67 ± 0.07 3 | 5.85 | 5.80 | 14.42 ± 0.04 | 15.66 | 15.04 | 10.96 ± 0.16 | 12.56 | 13.04 |
| 2 | 25 (−1) | 21 (−1) | 0.28 (+1) | 6.01 ± 0.04 | 6.13 | 6.09 | 14.63 ± 0.02 | 15.12 | 15.81 | 12.85 ± 0.03 | 13.45 | 15.81 |
| 3 | 25 (−1) | 43 (+1) | 0.18 (−1) | 6.03 ± 0.05 | 6.34 | 6.12 | 14.63 ± 0.02 | 15.90 | 15.97 | 12.85 ± 0.03 | 13.52 | 15.96 |
| 4 | 25 (−1) | 43 (+1) | 0.28 (+1) | 7.37 ± 0.03 | 7.21 | 7.56 | 16.94 ± 0.04 | 17.85 | 17.32 | 13.97 ± 0.03 | 12.75 | 13.57 |
| 5 | 75 (+1) | 21 (−1) | 0.18 (−1) | 6.02 ± 0.03 | 6.57 | 6.16 | 14.64 ± 0.04 | 14.12 | 15.45 | 12.85 ± 0.04 | 13.51 | 14.41 |
| 6 | 75 (+1) | 21 (−1) | 0.28 (+1) | 6.74 ± 0.05 | 6.61 | 6.87 | 16.44 ± 0.03 | 16.15 | 17.12 | 13.34 ± 0.03 | 14.87 | 14.57 |
| 7 | 75 (+1) | 43 (+1) | 0.18 (−1) | 7.27 ± 0.03 | 7.05 | 7.39 | 16.85 ± 0.02 | 18.32 | 17.86 | 13.87 ± 0.02 | 12.42 | 14.47 |
| 8 | 75 (+1) | 43 (+1) | 0.28 (+1) | 8.85 ± 0.05 | 8.93 | 9.86 | 20.76 ± 0.03 | 19.45 | 21.81 | 16.45 ± 0.04 | 17.03 | 17.22 |
| 9 | 50 (0) | 32 (0) | 0.23 (0) | 6.47 ± 0.05 | 6.04 | 6.57 | 16.86 ± 0.04 | 17.94 | 17.15 | 13.86 ± 0.02 | 15.12 | 17.15 |
| 10 | 50 (0) | 32 (0) | 0.23 (0) | 6.46 ± 0.03 | 6.13 | 6.49 | 16.87 ± 0.03 | 17.89 | 17.33 | 13.87 ± 0.04 | 15.09 | 17.26 |
| 11 | 0 (−α) | 32 (0) | 0.23 (0) | 5.81 ± 0.02 | 6.47 | 5.71 | 14.55 ± 0.03 | 15.64 | 15.12 | 12.46 ± 0.03 | 13.56 | 15.01 |
| 12 | 100 (+α) | 32 (0) | 0.23 (0) | 8.01 ± 0.04 | 7.80 | 7.57 | 16.95 ± 0.04 | 18.13 | 17.75 | 13.94 ± 0.03 | 13.51 | 15.86 |
| 13 | 50 (0) | 10 (−α) | 0.23 (0) | 5.99 ± 0.04 | 6.46 | 6.09 | 14.65 ± 0.04 | 13.90 | 15.14 | 12.85 ± 0.03 | 13.47 | 14.03 |
| 14 | 50 (0) | 54 (+α) | 0.23 (0) | 7.35 ± 0.03 | 7.79 | 7.37 | 16.93 ± 0.04 | 17.47 | 17.86 | 13.97 ± 0.03 | 15.29 | 15.87 |
| 15 | 50 (0) | 32 (0) | 0.148 (−α) | 5.57 ± 0.03 | 5.65 | 5.47 | 14.46 ± 0.03 | 15.66 | 15.12 | 11.94 ± 0.06 | 13.14 | 14.45 |
| 16 | 50 (0) | 32 (0) | 0.313 (+α) | 6.94 ± 0.02 | 7.11 | 6.89 | 17.03 ± 0.04 | 17.80 | 17.59 | 14.02 ± 0.04 | 15.26 | 14.87 |
| 17 | 50 (0) | 32 (0) | 0.23 (0) | 6.43 ± 0.03 | 6.01 | 6.56 | 16.86 ± 0.04 | 17.92 | 17.12 | 13.85 ± 0.02 | 15.07 | 17.11 |
| 18 | 50 (0) | 32 (0) | 0.23 (0) | 6.46 ± 0.03 | 6.09 | 6.52 | 16.85 ± 0.03 | 17.97 | 17.27 | 13.86 ± 0.04 | 15.13 | 17.04 |
| 19 | 50 (0) | 32 (0) | 0.23 (0) | 6.45 ± 0.02 | 6.03 | 6.51 | 16.86 ± 0.04 | 17.93 | 17.09 | 13.85 ± 0.02 | 15.11 | 17.09 |
| 20 | 50 (0) | 32 (0) | 0.23 (0) | 6.47 ± 0.03 | 6.11 | 6.54 | 16.87 ± 0.03 | 17.91 | 17.35 | 13.87 ± 0.04 | 15.16 | 17.21 |
| Y1: TE Yield (%) | Y2: ST Yield (mg/g) | Y3: Reb-A Yield (mg/g) | ||
|---|---|---|---|---|
| Source | DF | Estimated Coefficient | Estimated Coefficient | Estimated Coefficient |
| Model | 9 | 282.3273 ** | 119.727 * | 28.9583 ** |
| Intercept | ||||
| (β0) | 1 | 26.8068 ** | 43.1629 ** | 23.7821 ** |
| Linear terms | ||||
| X1 (β1) | 1 | −0.335019 ** | 0.390353 * | −0.685319 ** |
| X2 (β2) | 1 | 0.471612 ** | 0.499269 ** | −0.430433 ** |
| X3 (β3) | 1 | −0.602871 * | 0.619773 * | −0.471874 ** |
| Quadratic terms | ||||
| X12 (β11) | 1 | 0.352586 ** | 0.031327 ** | 0.070971 ** |
| X22 (β22) | 1 | −0.087273 ** | 0.088718 ** | −0.059163 ** |
| X32 (β33) | 1 | 0.080291 * | 0.075561 ** | 0.030346 ** |
| Interaction terms | ||||
| X1X2 (β1β2) | 1 | –0.301062 ** | −0.009164 ** | −0.002171 *** |
| X1X3 (β1β3) | 1 | 0.246349 ** | 0.008153 ** | 0.054853 ** |
| X2X3 (β2β3) | 1 | 0.874523 ** | 0.044789 ** | −0.001965 ** |
| Lack of fit(probability) | 7 | 0.0128 | 0.03561 | 0.00517 |
| F–value probability | <0.001 | <0.001 | <0.001 | |
| R2 | 0.9401 | 0.8874 | 0.9758 | |
| Adj. R2 | 0.8641 | 0.8419 | 0.9063 | |
| Predicted. R2 | 0.8396 | 0.8128 | 0.8363 |
| Y1: TE Yield (%) | Y2: ST Yield (mg/g) | Y3: Reb-A Yield (mg/g) | ||||
|---|---|---|---|---|---|---|
| Parameters | RSM | ANN | RSM | ANN | RSM | ANN |
| R2 (%) | 94.01 | 96.71 | 88.74 | 94.39 | 97.58 | 98.83 |
| RMSE | 3.72 | 2.05 | 4.53 | 1.58 | 6.54 | 1.71 |
| AAD (%) | 1.337 | 0.238 | 1.689 | 0.751 | 1.327 | 0.8137 |
| SEP (%) | 0.22 | 0.08 | 0.19 | 0.11 | 0.25 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameer, K.; Ameer, S.; Kim, Y.-M.; Nadeem, M.; Park, M.-K.; Murtaza, M.A.; Khan, M.A.; Nasir, M.A.; Mueen-ud-Din, G.; Mahmood, S.; et al. A Hybrid RSM-ANN-GA Approach on Optimization of Ultrasound-Assisted Extraction Conditions for Bioactive Component-Rich Stevia rebaudiana (Bertoni) Leaves Extract. Foods 2022, 11, 883. https://doi.org/10.3390/foods11060883
Ameer K, Ameer S, Kim Y-M, Nadeem M, Park M-K, Murtaza MA, Khan MA, Nasir MA, Mueen-ud-Din G, Mahmood S, et al. A Hybrid RSM-ANN-GA Approach on Optimization of Ultrasound-Assisted Extraction Conditions for Bioactive Component-Rich Stevia rebaudiana (Bertoni) Leaves Extract. Foods. 2022; 11(6):883. https://doi.org/10.3390/foods11060883
Chicago/Turabian StyleAmeer, Kashif, Saqib Ameer, Young-Min Kim, Muhammad Nadeem, Mi-Kyung Park, Mian Anjum Murtaza, Muhammad Asif Khan, Muhammad Adnan Nasir, Ghulam Mueen-ud-Din, Shahid Mahmood, and et al. 2022. "A Hybrid RSM-ANN-GA Approach on Optimization of Ultrasound-Assisted Extraction Conditions for Bioactive Component-Rich Stevia rebaudiana (Bertoni) Leaves Extract" Foods 11, no. 6: 883. https://doi.org/10.3390/foods11060883