Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
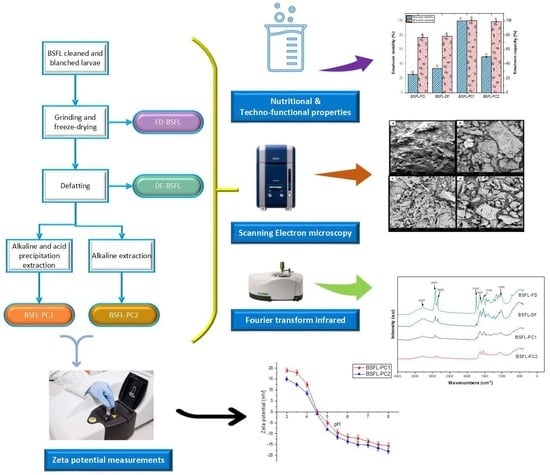
2.2. Edible Insects Flour Preparation
2.3. Preparation of Insect Proteins
2.3.1. Alkaline and Isoelectric Precipitation
2.3.2. Alkaline Extraction
2.4. Proximate Composition Analysis
2.5. Amino Acid Analysis
2.6. Bulk Density
2.7. Colour
2.8. Determination of Techno-Functional Properties
2.8.1. Water and Oil Binding Capacity
2.8.2. Emulsion Capacity and Stability
2.8.3. Solubility
2.8.4. Foaming Capacity and Stability
2.9. Surface Charge (Zeta Potential)
2.10. Scanning Electron Microscopy (SEM)
2.11. Fourier Transform Infrared Spectroscopy (FT-IR)
2.12. Data Analysis
3. Results and Discussion
3.1. Nutritional Properties
3.2. Bulk Density and Colour
3.3. Amino Acid Composition
3.4. Techno-Functional Properties
3.4.1. Water and Oil Binding Capacity
3.4.2. Emulsifying Capacity (EC) and Stability (ES)
3.4.3. Protein Solubility
3.4.4. Foaming Capacity and Foam Stability
3.4.5. Effect of pH on the ζ-Potential of BSFL Protein Concentrates
3.5. Scanning Electron Microscopy (SEM)
3.6. Fourier Transform Infrared (FT-IR) Spectrometer Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van-Huis, A.; Van-Itterbeeck, J.; Harmke, K.; Esther, M.; Afton, H.; Muir, M.; Paul, V. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Available online: http://edepot.wur.nl/258042 (accessed on 1 February 2020).
- Gmuer, A.; Guth, J.N.; Hartmann, C.; Siegrist, M. Effects of the degree of processing of insect ingredients in snacks on expected emotional experiences and willingness to eat. Food Qual. Prefer. 2016, 54, 117–127. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Can insects help to ease the problem of world food shortage. Search 1975, 6, 261–262. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Èntomol. 2013, 58, 563–583. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–24. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of house cricket (Acheta domesticus) flour addition on physicochemical and textural properties of meat emulsion under various formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef] [Green Version]
- La Barbera, F.; Verneau, F.; Amato, M.; Grunert, K. Understanding Westerners’ disgust for the eating of insects: The role of food neophobia and implicit associations. Food Qual. Prefer. 2018, 64, 120–125. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Barać, M.B.; Stanojević, S.P.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Šikoparija, B.; Radišić, P.; Prentović, M.; Pešić, M.B. Physicochemical composition and techno-functional properties of bee pollen collected in Serbia. LWT Food Sci. Technol. 2015, 62, 301–309. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Akpossan, R.A.; Digbeu, Y.D.; Koffi, M.D.; Kouadio, J.P.E.N.; Dué, E.A.; Kouamé, P.L. Protein fractions and functional properties of Dried Imbrasia oyemensis Larvae full-fat and defatted flours. Int. J. Biochem. Res. Rev. 2015, 5, 116–126. [Google Scholar] [CrossRef]
- Omotoso, O. Nutritional quality, functional properties and anti-nutrient compositions of the larva of Cirina forda (Westwood) (Lepidoptera: Saturniidae). J. Zhejiang Univ. Sci. B 2005, 7, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mshayisa, V.V.; Van Wyk, J. Hermetia illucens protein conjugated with glucose via maillard reaction: Antioxidant and techno-functional properties. Int. J. Food Sci. 2021, 2021, 5572554. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC, 15th ed.; Association of Official Agricultural Chemists: Arlington, VA, USA, 2015. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Diedericks, C.; Jideani, V.A. Physicochemical and functional properties of insoluble dietary fiber isolated from bambara groundnut (Vigna subterranean [L.] Verdc.). J. Food Sci. 2015, 80, C1933–C1944. [Google Scholar] [CrossRef] [PubMed]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.S.; Salas-Mellado, M.D.L.M. How extraction method affects the physicochemical and functional properties of chia proteins. LWT Food Sci. Technol. 2018, 96, 26–33. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT Food Sci. Technol. 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B.; Rodríguez, S.D. Structural properties of native and conjugated black soldier fly (Hermetia illucens) larvae protein via Maillard reaction and classification by SIMCA. Heliyon 2021, 7, e07242. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Helbig, A.; Wierenga, P.A.; Gruppen, H. Emulsion properties of algae soluble protein isolate from Tetraselmis sp. Food Hydrocoll. 2013, 30, 258–263. [Google Scholar] [CrossRef]
- Huang, C.; Feng, W.; Xiong, J.; Wang, T.; Wang, W.; Wang, C.; Yang, F. Impact of drying method on the nutritional value of the edible insect protein from black soldier fly (Hermetia illucens L.) larvae: Amino acid composition, nutritional value evaluation, in vitro digestibility, and thermal properties. Eur. Food Res. Technol. 2019, 245, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.; Landberg, R.; Langton, M. Yellow mealworm protein for food purposes—Extraction and functional properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [Green Version]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L.) cultivars. LWT Food Sci. Technol. 2013, 53, 278–284. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.-J.I.; Chen, J.; Benjamin, O. Extraction, characterization and functional properties of soluble proteins from edible grasshopper (Schistocerca gregaria) and honey bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
- Janssen, R.H.; Canelli, G.; Sanders, M.G.; Bakx, E.J.; Lakemond, C.M.M.; Fogliano, V.; Vincken, J.-P. Iron-polyphenol complexes cause blackening upon grinding Hermetia illucens (black soldier fly) larvae. Sci. Rep. 2019, 9, 2967. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Caligiani, A.; Sforza, S. Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: A metabolomics and proteomic insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Köhler, R.; Kariuki, L.; Lambert, C.; Biesalski, H. Protein, amino acid and mineral composition of some edible insects from Thailand. J. Asia Pac. Entomol. 2019, 22, 372–378. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAO Expert Consultation; FAO: Auckland, New Zealand, 2013; Available online: http://www.fao.org/ag/humannutrition/35978-02317b979a686a57aa4593304ffc17f06.pdf (accessed on 7 February 2022).
- Adebowale, Y.A.; Adebowale, K.O.; Oguntokun, M.O. Evaluation of nutritive properties of the large African Cricket (Gryllidae sp.). Pak. J. Sci. Ind. Res. 2005, 48, 274–278. [Google Scholar]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Rochow, V.; Gahukar, R.; Ghosh, S.; Jung, C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae, and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia-Pac. Èntomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition. Report of an FAO Expert Consultation; FAO: Geneva, Switzerland, 2007. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14258992 (accessed on 8 January 2019).
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Ndiritu, A.K.; Kinyuru, J.N.; Kenji, G.M.; Gichuhi, P.N. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2017, 11, 2013–2021. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Lopez-Alonso, M.; Hayes, M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) S. F. Gray. Food Res. Int. 2017, 99, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Y.; Xie, W.; Zhou, D.; Tang, S.; Kuang, M.; Wang, Y.; Du, S.-K. Physicochemical and functional properties of protein isolate obtained from cottonseed meal. Food Chem. 2018, 240, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, O.T. An evaluation of the nutrients and some anti-nutrients in Silkworm, Bombyxmori L. (Bombycidae: Lepidoptera). Jordan J. Biol. Sci. 2015, 8, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Schwenzfeier, A.; Wierenga, P.A.; Eppink, M.; Gruppen, H. Effect of charged polysaccharides on the techno-functional properties of fractions obtained from algae soluble protein isolate. Food Hydrocoll. 2014, 35, 9–18. [Google Scholar] [CrossRef]
- Janssen, R.H. Potential of Insect Proteins for Food and Feed: Effect of Endogenous Enzymes and Iron-Phenolic Complexation. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2018; pp. 1–157. [Google Scholar] [CrossRef] [Green Version]
- Hadnađev, M.; Dapčević-Hadnađev, T.; Lazaridou, A.; Moschakis, T.; Michaelidou, A.-M.; Popović, S.; Biliaderis, C. Hempseed meal protein isolates prepared by different isolation techniques. Part I. physicochemical properties. Food Hydrocoll. 2018, 79, 526–533. [Google Scholar] [CrossRef]
- Wang, W.-D.; Li, C.; Bin, Z.; Huang, Q.; You, L.-J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef]









| Sample | Crude Protein (%) | Crude Fat (%) | Carbohydrates (%) | Moisture (%) | Ash (%) |
|---|---|---|---|---|---|
| BSFL-FD | 44.47 ± 1.77 a | 22.60 ± 1.39 b | 21.13 ± 0.57 a | 9.48 ± 0.34 c | 10.81 ± 0.26 d |
| BSFL-DF | 50.12 ± 0.66 b | 0.83 ± 0.06 a | 40.80 ± 0.39 d | 6.07 ± 0.25 b | 8.25 ± 0.33 c |
| BSFL-PC1 | 73.35 ± 0.88 d | 0.37 ± 0.12 a | 22.92 ± 1.02 b | 1.48 ± 0.01 a | 3.36 ±0.28 b |
| BSFL-PC2 | 68.47 ± 0.93 c | 0.27 ± 0.06 a | 29.19 ± 0.92 c | 1.57 ± 0.03 a | 2.08 ±0.01 a |
| Sample | Bulk Density (g/mL) | L * | a * | b * | ΔE |
|---|---|---|---|---|---|
| BSFL-FD | 1.01 ± 0.02 b | 51.24 ± 0.34 c | 0.84 ± 1.34 ab | 17.99 ± 1.70 a | Control |
| BSFL-DF | 1.04 ± 0.02 b | 62.95 ± 1.01 d | 1.67 ± 0.12 b | 18.43 ± 2.67 a | 11.95 ± 1.16 a |
| BSFL-PC1 | 0.84 ± 0.01 a | 44.56 ± 0.63 b | 0.85 ± 1.35 ab | 17.99 ± 1.70 a | 16.16 ± 1.27 a |
| BSFL-PC2 | 0.86 ± 0.01 a | 35.20 ± 1.87 a | −2.14 ± 3.28 a | 18.78 ± 6.56 a | 23.87 ± 4.16 b |
| Amino Acid | BSFL-FD | BSFL-DF | BSFL-PC1 | BSFL-PC2 | Cow’s Milk | Egg Protein | FAO [36] |
|---|---|---|---|---|---|---|---|
| Essential | |||||||
| Histidine | 1.82 ± 0.01 a | 3.84 ± 0.01 d | 3.64 ± 0.02 c | 2.48 ± 0.00 b | 2.70 | 2.40 | 1.50 |
| Isoleucine | 2.49 ± 0.01 a | 4.32 ± 0.44 bc | 5.18 ± 0.78 c | 3.99 ± 0.45 b | 4.90 | 5.60 | 3.00 |
| Leucine | 4.01 ± 0.12 a | 7.29 ± 0.15 c | 7.99 ± 0.29 d | 4.61 ± 0.17 b | 9.10 | 8.30 | 5.90 |
| Lysine | 3.73 ± 0.01 a | 6.79 ± 0.19 c | 9.16 ± 0.34 d | 5.37 ± 0.20 b | 7.40 | 6.30 | 4.5 |
| Methionine | 2.53 ± 0.00 a | 1.72 ± 0.45 a | 2.53 ± 0.00 a | 2.67 ± 0.08 a | 2.60 | 3.20 | 1.60 |
| Phenylalanine | 4.14 ± 0.01 a | 4.36 ± 0.20 a | 7.18 ± 0.05 c | 5.41 ± 0.21 b | 4.90 | 5.10 | Not supplied |
| Threonine | 2.87 ± 0.53 a | 4.09 ± 0.49 b | 4.95 ± 0.12 bc | 4.65 ± 0.00 b | 4.40 | 5.10 | 2.30 |
| Valine | 3.39 ± 0.01 a | 5.80 ± 0.51 b | 5.61 ± 0.89 c | 5.09 ± 0.51 b | 6.60 | 7.60 | 3.90 |
| Sum | 24.98 ± 0.80 a | 38.20 ± 2.44 b | 46.52 ± 2.48 c | 34.27 ± 2.19 b | 42.60 | 43.60 | 22.70 |
| Non-essential | |||||||
| Alanine | 3.58 ± 0.01 a | 5.95 ± 0.48 c | 4.66 ± 0.04 b | 5.41 ± 0.48 bc | 3.60 | 5.40 | - |
| Arginine | 3.38 ± 0.00 a | 4.85 ± 0.36 b | 4.57 ± 0.02 b | 5.72 ± 0.02 c | 3.60 | 6.10 | - |
| Aspartic acid | 5.89 ± 0.01 a | 10.38 ± 0.39 b | 12.56 ± 0.69 c | 9.62 ± 0.40 a | 7.70 | 10.70 | - |
| Glycine | 3.08 ± 0.06 a | 4.80 ± 0.13 c | 3.88 ± 0.06 b | 4.97 ± 0.15 c | 3.00 | 2.00 | - |
| Glutamic acid | 6.89 ± 0.05 a | 13.62 ± 0.05 d | 12.13 ± 0.09 b | 12.40 ± 0.05 c | 12.0 | 20.60 | - |
| Proline | 2.57 ± 0.01 a | 5.71 ± 0.23 d | 4.38 ± 0.01 c | 3.38 ± 0.24 b | 8.50 | 3.80 | - |
| Serine | 2.13 ± 0.04 a | 4.42 ±0.10 d | 3.99 ± 0.28 c | 3.13 ± 0.17 b | 5.20 | 7.90 | - |
| Tyrosine | 6.89 ± 0.00 c | 6.47 ± 0.07 b | 6.28 ± 0.15 a | 9.34 ± 0.09 b | 4.10 | 7.60 | - |
| Sum | 34.41 ± 0.21 a | 56.21 ± 1.74 b | 52.45 ± 1.85 b | 53.98 ± 2.00 b | 55.30 | 56.50 | - |
| Amino Acids | FAO/WHO/UNU 2007 [41] (mg/g Protein) | Chemical Score (%) | |||
|---|---|---|---|---|---|
| BSFL-FD | BSFL-DF | BSFL-PC1 | BSFL-PC2 | ||
| Histidine | 15 | 191.84 | 254.03 | 175.72 | 230.52 |
| Isoleucine | 30 | 139.86 | 152.62 | 150.66 | 174.96 |
| Leucine | 59 | 110.73 b | 126.51 c | 85.67 b | 132.72 b |
| Lysine | 45 | 130.74 c | 149.90 | 126.75 c | 193.37 |
| Methionine | 16 | 265.73 | 113.68 b | 188.91 | 160.22 |
| Threonine | 23 | 193.08 | 173.10 | 210.92 | 200.63 |
| Valine | 39 | 142.76 | 153.46 | 144.30 | 142.11 c |
| Phenylalanine + tyrosine | 38 | 488.83 | 301.99 | 439.71 | 358.91 |
| Total EAA | 277 | 151.8 | 146.1 | 140.2 | 169.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. https://doi.org/10.3390/foods11050724
Mshayisa VV, Van Wyk J, Zozo B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods. 2022; 11(5):724. https://doi.org/10.3390/foods11050724
Chicago/Turabian StyleMshayisa, Vusi Vincent, Jessy Van Wyk, and Bongisiwe Zozo. 2022. "Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates" Foods 11, no. 5: 724. https://doi.org/10.3390/foods11050724