Asparagus Roots: From an Agricultural By-Product to a Valuable Source of Fructans
Abstract
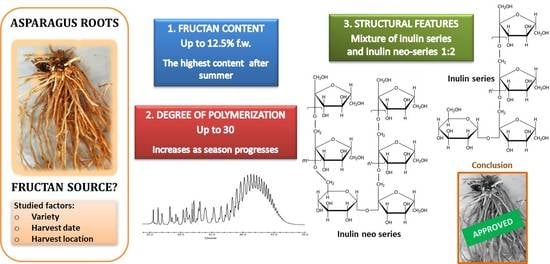
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fructan Extraction
2.3. Purification and Fractionation of Asparagus Fructans
2.4. Study of Average Molecular Weight Distribution by Gel Filtration
2.5. Study of Polymerization Degree of the Different Fructan Fraction by HPAEC
2.6. Study of Fructan Structure by Methylation (GC-MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Contents in Neutral Sugars and Fructans
3.2. Fructans Fractionation
3.3. Molecular Weight Distribution and Structural Features
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeasmin, R.; Motoki, S.; Yamamoto, S.; Nishihara, E. Allelochemicals Inhibit the Growth of Subsequentely Replanted Asparagus (Asparagus officinalis L.). Biol. Agric. Hortic. 2013, 29, 165–172. [Google Scholar] [CrossRef]
- Elmer, W.H. Management of Fusarium Crown and Root Rot of Asparagus. Crop Prot. 2015, 73, 2–6. [Google Scholar] [CrossRef]
- FAOSTAT On-Line Statistical Database of the Food and Agricultural Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#home (accessed on 27 October 2021).
- Shiomi, N. Two Novel Hexasaccharides from the Roots of Asparagus officinalis. Phytochemistry 1981, 20, 2581–2583. [Google Scholar] [CrossRef]
- Shiomi, N.; Yamada, J.; Izawa, M. Isolation and Identification of Fructo-Oligosaccharides in Roots of Asparagus (Asparagus officinalis L.). Agric. Biol. Chem. 1976, 40, 567–575. [Google Scholar] [CrossRef]
- Shiomi, N.; Yamada, J.; Izawa, M. A Novel Pentasaccharide in the Roots of Asparagus (Asparagus officinalis L.). Agric. Biol. Chem. 1979, 43, 1375–1377. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Alventosa, J.M.; Jaramillo-Carmona, S.; Rodríguez-Gutiérrez, G.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Effect of Extraction Method on Phytochemical Composition and Antioxidant Activity of High Dietary Fibre Powders Obtained from Asparagus By-Products. Food Chem. 2009, 116, 484–490. [Google Scholar] [CrossRef]
- Viera-Alcaide, I.; Hamdi, A.; Rodríguez-Arcos, R.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Asparagus Cultivation Co-Products: From Waste to Chance. J. Food Sci. Nutr. 2020, 6, 057. [Google Scholar] [CrossRef]
- Shiomi, N. Content of Carbohydrate and Activities of Fructosyltransferase and Invertase in Asparagus Roots during the Fructo-Oligosaccharide- and Fructo-Polysaccharide-Accumulating Season. New Phytol. 1992, 122, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Maeda, T.; Nomura, S.; Suzuki, M.; Grant, G.; Sporns, P. Rapid Analysis of Fructans and Comparison of Fructans Profiles in Several Different Types of Asparagus Storage Roots Using MALDI-TOF MS. J. Hortic. Sci. Biotech. 2011, 86, 210–216. [Google Scholar] [CrossRef]
- Witzel, K.; Matros, A. Fructans Are Differentially Distributed in Root Tissues of Asparagus. Cells 2020, 9, 1943. [Google Scholar] [CrossRef]
- Yoshida, M. Fructan Structure and Metabolism in Overwintering Plants. Plants 2021, 10, 933. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Fructan Metabolism in A. Tequilana Weber Blue Variety along Its Developmental Cycle in the Field. J. Agric. Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef]
- Nobre, C.; Teixeira, J.A.; Rodrigues, L.R. New Trends and Technological Challenges in the Industrial Production and Purification of Fructo-Oligosaccharides. Crit. Rev. Food Sci. 2015, 55, 1444–1455. [Google Scholar] [CrossRef]
- Verma, D.K.; Patel, A.R.; Thakur, M.; Singh, S.; Tripathy, S.; Srivastav, P.P.; Chávez-González, M.L.; Gupta, A.K.; Aguilar, C.N. A Review of the Composition and Toxicology of Fructans, and Their Applications in Foods and Health. J. Food Compos. Anal. 2021, 99, 103884. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Inulin-Type Fructans: Functional Food Ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, Health Benefits and Food Applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Recent Trends in the Microbial Production, Analysis and Application of Fructooligosaccharides. Trends Food Sci. Technol. 2005, 16, 442–457. [Google Scholar] [CrossRef]
- Climate data Climate Data for Cities Worldwide—Climate-Data.Org. Available online: https://en.climate-data.org/ (accessed on 29 October 2021).
- Moreno, R.; Espejo, J.A.; Cabrera, A.; Gil, J. Origin of Tetraploid Cultivated Asparagus Landraces Inferred from Nuclear Ribosomal DNA Internal Transcribed Spacers’ Polymorphisms. Ann. Appl. Biol. 2008, 153, 233–241. [Google Scholar] [CrossRef]
- Dische, Z. Color Reactions of Carbohydrates. In Methods in Carbohydrate Chemistry; Whistler, R.L., Wolfram, M.L., Eds.; Academic Press: New York, NY, USA, 1962; Volume 1, pp. 477–512. [Google Scholar]
- Megazyme Fructan Assay Kit—Measurement of Fructan in Plants Food|Megazyme. Available online: https://www.megazyme.com/fructan-assay-kit (accessed on 27 October 2021).
- Dos-Santos, N.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Fernández-Trujillo, J.P. Cell Wall Polysaccharides of Near-Isogenic Lines of Melon (Cucumis melo L.) and Their Inbred Parentals Which Show Diferential Flesh Firmness or Physiological Behavior. J. Agric. Food Chem. 2011, 59, 7773–7784. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo-Carmona, S.M.; Javier Tejero-Maján, F.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R. Comparative Analysis of Chemical Compounds Related to Quality of Canned Asparagus. J. Food Nutr. Res. 2019, 7, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Beneo Inulin from Orafti®|BENEO Prebiotic, Dietary Chicory Root Fibre. Available online: https://www.beneo.com/ingredients/human-nutrition/functional-fibres/inulin (accessed on 27 October 2021).
- Hakomori, A. A Rapid Permethylation of Glycolipid and Polysaccharide Catalyzed by Methylsulfinyl Carbanion in Dimethyl Sulfoxide. J. Biochem. 1964, 65, 205–208. [Google Scholar]
- Kvernheim, A. Methylation Analysis of Polysaccharides with Butyllithium in Dimethyl Sulfoxide. Acta Chem. Scand. B 1987, 41, 150–152. [Google Scholar] [CrossRef] [Green Version]
- York, W.S.; Darvill, A.G.; McNeil, M.; Stevenson, T.T.; Albersheim, P. Isolation and Characterization of Plant Cell Walls and Cell Wall Components. In Methods in Enzymology 118: Plant Molecular Biology; Weissbach, A., Weissbach, H., Eds.; Plant Molecular Biology; Academic Press: New York, NY, USA, 1986; pp. 3–40. [Google Scholar]
- Carpita, N.C.; Shea, A.M. Linkage Structure of Carbohydrates by Gas Chromatography-Mass Spectrometry of Partially Methylated Alditol Acetates. In Analysis of Carbohydrates by GLC and MS; Biermann, C.J., McGinnis, G.D., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 157–216. [Google Scholar]
- Puangbut, D.; Jogloy, S.; Vorasoot, N.; Srijaranai, S.; Holbrook, C.C.; Patanothai, A. Variation of Inulin Content, Inulin Yield and Water Use Efficiency for Inulin Yield in Jerusalem Artichoke Genotypes under Different Water Regimes. Agric. Water Manag. 2015, 152, 142–150. [Google Scholar] [CrossRef]
- Mathieu, A.-S.; Tinel, C.; Dailly, H.; Quinet, M.; Lutts, S. Impact of High Temperature on Sucrose Translocation, Sugar Content and Inulin Yield in Cichorium intybus L. Var. Sativum. Plant Soil 2018, 432, 273–288. [Google Scholar] [CrossRef]
- Flores-Girón, E.; Salazar-Montoya, J.A.; Ramos-Ramírez, E.G. Application of a Box-Behnken Design for Optimizing the Extraction Process of Agave Fructans (Agave Tequilana Weber Var. Azul): Box-Behnken Design for Optimizing the Extraction Process of Agave Fructans. J. Sci. Food Agric. 2016, 96, 3860–3866. [Google Scholar] [CrossRef]
- Aldrete-Herrera, P.I.; López, M.G.; Medina-Torres, L.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-Ávila, M.; Ortiz-Basurto, R.I. Physicochemical Composition and Apparent Degree of Polymerization of Fructans in Five Wild Agave Varieties: Potential Industrial Use. Foods 2019, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- McCallum, J.; Clarke, A.; Pither-Joyce, M.; Shaw, M.; Butler, R.; Brash, D.; Scheffer, J.; Sims, I.; van Heusden, S.; Shigyo, M.; et al. Genetic Mapping of a Major Gene Affecting Onion Bulb Fructan Content. Theor. Appl. Genet. 2006, 112, 958–967. [Google Scholar] [CrossRef]
- Khaldari, I.; Naghavi, M.R.; Peighambari, S.A.; Nasiri, J.; Mohammadi, F. Expression Patterns of the Genes Encoding Fructan Active Enzymes (FAZYs) alongside Fructan Constituent Profiles in Chicory (Cichorium intybus L.): Effects of Tissue and Genotype Variations. J. Plant Biochem. Biotechnol. 2018, 27, 453–462. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez, G.; Cermeño, P.; Jiménez, A.; Guillén, R.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Identification of Flavonoid Diglycosides in Several Genotypes of Asparagus from the Huétor-Tájar Population Variety. J. Agric. Food Chem. 2007, 55, 10028–10035. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Jaramillo, S.; Rodríguez-Gutiérrez, G.; Cermeño, P.; Espejo, J.A.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Fernández-Bolaños, J.; Rodríguez-Arcos, R. Flavonoid Profile of Green Asparagus Genotypes. J. Agric. Food Chem. 2008, 56, 6977–6984. [Google Scholar] [CrossRef]
- Vázquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Cermeño-Sacristán, P.; Espejo-Calvo, J.A.; Guillén-Bejarano, R. Saponin Profile of Green Asparagus Genotypes. J. Agric. Food Chem. 2013, 61, 11098–11108. [Google Scholar] [CrossRef]
- Vázquez-Castilla, S.; Jaramillo-Carmona, S.; Fuentes-Alventosa, J.M.; Jiménez-Araujo, A.; Rodríguez-Arcos, R.; Cermeño-Sacristán, P.; Espejo-Calvo, J.A.; Guillén-Bejarano, R. Optimization of a Method for the Pofiling and Quantification of Saponins in Different Green Asparagus Genotypes. J. Agric. Food Chem. 2013, 61, 6250–6258. [Google Scholar] [CrossRef]
- Saengthongpinit, W.; Sajjaanantakul, T. Influence of Harvest Time and Storage Temperature on Characteristics of Inulin from Jerusalem Artichoke (Helianthus tuberosus L.) Tubers. Postharvest Biol. Technol. 2005, 37, 93–100. [Google Scholar] [CrossRef]
- De Roover, J.; Vandenbranden, K.; Van Laere, A.; Van den Ende, W. Drought Induces Fructan Synthesis and 1-SST (Sucrose: Sucrose Fructosyltransferase) in Roots and Leaves of Chicory Seedlings (Cichorium intybus L.). Planta 2000, 210, 808–814. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Plant Fructans in Stress Environments: Emerging Concepts and Future Prospects. J. Exp. Bot. 2008, 59, 2905–2916. [Google Scholar] [CrossRef] [Green Version]
- López-Molina, D.; Navarro-Martínez, M.D.; Rojas Melgarejo, F.; Hiner, A.N.P.; Chazarra, S.; Rodríguez-López, J.N. Molecular Properties and Prebiotic Effect of Inulin Obtained from Artichoke (Cynara scolymus L.). Phytochemistry 2005, 66, 1476–1484. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. BioMed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [Green Version]
- Monti, A.; Amaducci, M.T.; Pritoni, G.; Venturi, G. Growth, Fructan Yield, and Quality of Chicory (Cichorium intybus L.) as Related to Photosynthetic Capacity, Harvest Time, and Water Regime. J. Exp. Bot. 2005, 56, 1389–1395. [Google Scholar] [CrossRef] [Green Version]
- Shiomi, N. Structure of Fructopolysaccharide (Asparagosin) from Roots of Asparagus (Asparagus officinalis L.). New Phytol. 1993, 123, 263–270. [Google Scholar] [CrossRef]
- Matros, A.; Houston, K.; Tucker, M.R.; Schreiber, M.; Berger, B.; Aubert, M.K.; Wilkinson, L.G.; Witzel, K.; Waugh, R.; Seiffert, U.; et al. Genome-Wide Association Study Reveals the Genetic Complexity of Fructan Accumulation Patterns in Barley Grain. J. Exp. Bot. 2021, 72, 2383–2402. [Google Scholar] [CrossRef]
- Thakur, M.; Connellan, P.; Deseo, M.A.; Morris, C.; Praznik, W.; Loeppert, R.; Dixit, V.K. Characterization and in Vitro Inmunomodulatory Screening of Fructo-Oligosaccharides of Asparagus Racemosus Willd. Int. J. Biol. Macromol. 2012, 50, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S. The Metabolism of Fructooligosaccharides and Fructooligosaccharide-Related Compounds. Food Rev. Int. 2010, 27, 16–50. [Google Scholar] [CrossRef]




| Location | Variety | Species | Sampling Date |
|---|---|---|---|
| Chipiona (Cádiz) CA | Herkolim HK | A. officinalis | June S1 September S2 December S3 |
| Primems PM | A. officinalis | ||
| Huétor-Tájar (Granada) GR | Grande GD | A. officinalis | |
| Huétor-Tájar landrace HT | A. officinalis × A. maritimus |
| S1 | S2 | S3 | |
|---|---|---|---|
| Herkolim | 13.41 ± 0.99 c | 26.58 ± 1.75 g | 8.34 ± 0.76 a |
| Primens | 14.84 ± 0.73 d | 11.23 ± 0.81 b | 15.60 ± 1.11 de |
| Grande | 16.44 ± 1.30 e | 8.27 ± 0.69 a | 15.95 ± 1.39 de |
| Huétor-Tájar landrace | 11.69 ± 0.50 b | 10.49 ± 0.85 b | 18.89 ± 1.80 f |
| S1 | S2 | S3 | |
|---|---|---|---|
| Herkolim | 6.36 ± 0.20 f 47.47 | 12.53 ± 0.44 h 47.13 | 5.73 ± 0.21 e 68.70 |
| Primens | 3.08 ± 0.30 bc 20.76 | 3.41 ± 0.23 c 30.37 | 2.87 ± 0.21 b 18.41 |
| Grande | 5.33 ± 0.49 e 32.43 | 4.80 ± 0.39 d 58.07 | 6.27 ± 0.56 f 39.33 |
| Huétor-Tájar landrace | 0.92 ± 0.08 a 7.90 | 4.70 ± 0.22 d 44.81 | 8.02 ± 0.30 g 42.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viera-Alcaide, I.; Hamdi, A.; Guillén-Bejarano, R.; Rodríguez-Arcos, R.; Espejo-Calvo, J.A.; Jiménez-Araujo, A. Asparagus Roots: From an Agricultural By-Product to a Valuable Source of Fructans. Foods 2022, 11, 652. https://doi.org/10.3390/foods11050652
Viera-Alcaide I, Hamdi A, Guillén-Bejarano R, Rodríguez-Arcos R, Espejo-Calvo JA, Jiménez-Araujo A. Asparagus Roots: From an Agricultural By-Product to a Valuable Source of Fructans. Foods. 2022; 11(5):652. https://doi.org/10.3390/foods11050652
Chicago/Turabian StyleViera-Alcaide, Isabel, Amel Hamdi, Rafael Guillén-Bejarano, Rocío Rodríguez-Arcos, Juan Antonio Espejo-Calvo, and Ana Jiménez-Araujo. 2022. "Asparagus Roots: From an Agricultural By-Product to a Valuable Source of Fructans" Foods 11, no. 5: 652. https://doi.org/10.3390/foods11050652