Identification of Benzophenone Analogs in Rice Cereal through Fast Pesticide Extraction and Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Experiment
2.1. Reagents and Chemicals
2.2. Standard
2.3. Sample Treatment
2.4. Instrumentation
2.5. Method Validation Procedure and Real Sample Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Cleanup Method Selection
3.2. Method Validation
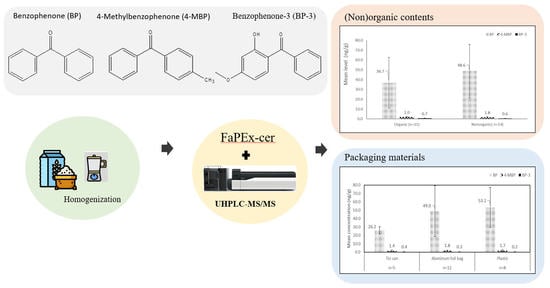
3.3. Sample Analysis
3.4. Risk Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maslova, G.M.; Glinkina, I.M.; Kashirina, N.A.; Bailova, N.V. Market research of rice cereals. IOP Conf. Ser. Earth Environ. Sci. 2020, 422, 012117. [Google Scholar] [CrossRef]
- FAO Cereal Supply and Demand Brief. FAO Cereal Supply and Demand Brief, World Food Situation, 2021. FAO Cereal Supply and Demand Brief, World Food Situation, Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/worldfoodsituation/csdb/en/ (accessed on 12 October 2021).
- Darbre, P.D. The history of endocrine-disrupting chemicals. Curr. Opin. Endocr. Metab. Res. 2019, 7, 26–33. [Google Scholar] [CrossRef]
- IARC. Benzophenone. In Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water; International Agency for Research on Cancer: Lyon, France, 2013; pp. 285–301. [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of benzophenone (CAS No. 119-61-9) in F344/N rats and B6C3F1 mice (feed studies). Natl. Toxicol. Program Tech. Rep. Ser. 2006, 533, 1–264. [Google Scholar]
- EFSA. Toxicological evaluation of benzophenone. EFSA 2009, 7, 1–30. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitamura, S.; Khota, R.; Sugihara, K.; Fujimoto, N.; Ohta, S. Estrogenic and antiandrogenic activities of 17 benzophenone derivatives used as UV stabilizers and sunscreens. Toxicol. Appl. Pharm. 2005, 203, 9–17. [Google Scholar] [CrossRef]
- Jiménez-Díaz, I.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. Analytical methods for the determination of personal care products in human samples: An overview. Talanta 2014, 129, 448–458. [Google Scholar] [CrossRef]
- EFSA. EFSA statement on the presence of 4-methylbenzophenone found in breakfast cereals. EFSA J. 2009, 7, 243r. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic Materials and Articles Intended to Come into Contact with Food Text with EEA Relevance; European Commission: Brussels, Belgium, 2011; Volume 45, pp. 42–130. [Google Scholar]
- Tsochatzis, E.D.; Alberto Lopes, J.; Hoekstra, E.; Emons, H. Development and validation of a multi-analyte GC-MS method for the determination of 84 substances from plastic food contact materials. Anal. Bioanal. Chem. 2020, 412, 5419–5434. [Google Scholar] [CrossRef]
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Benzophenones. Dermatitis 2014, 25, 3–10. [Google Scholar] [CrossRef]
- Wang, L.; Asimakopoulos, A.G.; Moon, H.-B.; Nakata, H.; Kannan, K. Benzotriazole, benzothiazole, and benzophenone compounds in indoor dust from the United States and East Asian countries. Environ. Sci. Technol. 2013, 47, 4752–4759. [Google Scholar] [CrossRef]
- Hayden, C.G.; Roberts, M.S. Systemic absorption of sunscreen after topical application. Lancet 1997, 350, 863–864. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Barreca, S.; Forni, C.; Colzani, L.; Clerici, L.; Daverio, D.; Dellavedova, P. Study on the Stability of Antibiotics, Pesticides and Drugs in Water by Using a Straightforward Procedure Applying HPLC-Mass Spectrometric Determination for Analytical Purposes. Separations 2021, 8, 179. [Google Scholar] [CrossRef]
- Jung, T.; Simat, T.J.; Altkofer, W.; Fugel, D. Survey on the occurrence of photo-initiators and amine synergists in cartonboard packaging on the German market and their migration into the packaged foodstuffs. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess 2013, 30, 1993–2016. [Google Scholar] [CrossRef]
- Bugey, A.; Janin, Y.; Edder, P.; Bieri, S. Targeted multidimensional gas chromatography using a heart-cutting device and cryogenic focusing for the determination of benzophenone derivatives in foodstuffs. Anal. Bioanal. Chem. 2013, 405, 4177–4185. [Google Scholar] [CrossRef]
- Van Hoeck, E.; De Schaetzen, T.; Pacquet, C.; Bolle, F.; Boxus, L.; Van Loco, J. Analysis of benzophenone and 4-methylbenzophenone in breakfast cereals using ultrasonic extraction in combination with gas chromatography-tandem mass spectrometry (GC-MS(n)). Anal. Chim. Acta 2010, 663, 55–59. [Google Scholar] [CrossRef]
- Sun, C.; Chan, S.H.; Lu, D.; Lee, H.M.; Bloodworth, B.C. Determination of isopropyl-9H-thioxanthen-9-one in packaged beverages by solid-phase extraction clean-up and liquid chromatography with tandem mass spectrometry detection. J. Chromatogr. A 2007, 1143, 162–167. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Colzani, L.; Clerici, L.; Daverio, D.; Dellavedova, P.; Balzamo, S.; Calabretta, E.; Ubaldi, V. Determination of estrogenic endocrine disruptors in water at sub-ng L− 1 levels in compliance with Decision 2015/495/EU using offline-online solid phase extraction concentration coupled with high performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 147, 1186–1191. [Google Scholar] [CrossRef]
- Chang, H.C.; Chen, Y.J.; Chang, M.H.; Liao, C.D.; Kao, Y.M.; Wang, D.Y.; Cheng, H.F. Novel multi-analyte method for detection of thirty photoinitiators in breakfast cereal and packaged juice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1130–1131, 121788. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Nunez, O.; Moyano, E.; Galceran, M.T. Analysis of UV ink photoinitiators in packaged food by fast liquid chromatography at sub-ambient temperature coupled to tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 459–466. [Google Scholar] [CrossRef] [Green Version]
- Chuang, W.C.; Chen, J.W.; Huang, C.H.; Shyu, T.H.; Lin, S.K. FaPEx® Multipesticide Residues Extraction Kit for Minimizing Sample Preparation Time in Agricultural Produce. J. AOAC Int. 2019, 102, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Pastorelli, S.; Cruz, J.M.; Simoneau, C.; Castanheira, I.; Paseiro-Losada, P. Development of an analytical method for the determination of photoinitiators used for food packaging materials with potential to migrate into milk. J. Dairy Sci. 2008, 91, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivikko, R.; Pastorelli, S.; Rodriguez-Bernaldo de Quiros, A.; Paseiro-Cerrato, R.; Paseiro-Losada, P.; Simoneau, C. Rapid multi-analyte quantification of benzophenone, 4-methylbenzophenone and related derivatives from paperboard food packaging. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess 2010, 27, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Van Den Houwe, K.; Van Heyst, A.; Evrard, C.; Van Loco, J.; Bolle, F.; Lynen, F.; Van Hoeck, E. Migration of 17 Photoinitiators from Printing Inks and Cardboard into Packaged Food—Results of a Belgian Market Survey. Packag. Technol. Sci. 2016, 29, 121–131. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chien, J.T.; Chen, H.C.; Liu, X.R.; Chang, J.P.; Huang, J.J. Rapid determination of benzophenone derivatives in cereals using FaPEx coupled with ultra–high-performance liquid chromatography–tandem mass spectrometry. J. Food Drug Anal. 2021, 29, 287–302. [Google Scholar] [CrossRef]
- International Food Standards. Codex Alimentarius. Guidelines on Performance Criteria for Methods of Analysis for the Determination of Pesticide Residues in Food and Feed; CCPR, 2017; CXG90. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B90-2017%252FCXG_090e.pdf (accessed on 15 October 2021).
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018, 410, 3789–3803. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef] [Green Version]
- EPA, U.S. Human Health Risk Assessment Protocol. In Chapter 7 Characterizing Risk and Hazard; EPA, U.S.: Washington, DC, USA, 2005. [Google Scholar]
- TFDA. National Food Consumption Database. 2019. Available online: http://tnfcds.cmu.edu.tw (accessed on 22 August 2020).
- EFSA. Polycyclic Aromatic Hydrocarbons in Food-Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 724. [Google Scholar] [CrossRef]





| Mass Spectrometer | Triple Quadrupole Shimadzu Tandem MS (Shimadzu–8045) | ||||||
|---|---|---|---|---|---|---|---|
| Ionization | Electrospray Ionization, ESI (Multiple Reaction Monitoring Mode) | ||||||
| No. | Analytes | Quantification | Qualification | RT (min) | CE 1(v) | CE2 (v) | ESI |
| 1 | BP-2 | 245.0 > 135.0 | 245.0 > 109.0 | 5.64 | −12 | −16 | - |
| 2 | d4-4OHBP | 203.1 > 125.2 | 203.1 > 105.1 | 6.19 | 23 | 23 | + |
| 3 | 4-OHBP | 199.0 > 121.1 | 199.0 > 77.1 | 6.20 | 10 | 10 | + |
| 4 | M2BB | 240.3 > 209.1 | 240.3 > 152.0 | 6.37 | 17 | 17 | + |
| 5 | d5-BP1 | 220.1 > 137.0 | 220.1 > 138.0 | 6.59 | 25 | 25 | + |
| 6 | BP-1 | 214.9 > 137.0 | 214.9 > 105.0 | 6.61 | 23 | 15 | + |
| 7 | d3-BP-8 | 248.1 > 121.1 | 248.1 > 154.1 | 6.77 | 28 | 30 | + |
| 8 | BP-8 | 245.0 > 121.1 | 245.0 > 151.0 | 6.79 | 13 | 13 | + |
| 9 | d5-BP | 188.1 > 105.1 | 188.1 > 110.1 | 6.80 | 21 | 22 | + |
| 10 | BP | 183.0 > 105.1 | 183.0 > 77.2 | 6.83 | 19 | 19 | + |
| 11 | 13C6-di-OHBP | 221.0 > 137.0 | 221.0 > 81.0 | 6.61 | 11 | 24 | + |
| 12 | 2-OHBP | 199.2 > 121.0 | 199.2 > 93.0 | 7.22 | 11 | 11 | + |
| 13 | d3-4-MBP | 200.2 > 105.1 | 200.2 > 77.1 | 7.31 | 12 | 12 | + |
| 14 | 4-MBP | 197.0 > 105.1 | 197.0 > 77.1 | 7.33 | 21 | 10 | + |
| 15 | d5-BP3 | 234.0 > 151.0 | 234.1 > 82.0 | 7.51 | 27 | 26 | + |
| 16 | BP-3 | 229.0 > 151.1 | 229.0 > 105.1 | 7.54 | 25 | 11 | + |
| 17 | PBZ | 259.0 > 105.0 | 259.0 > 77.1 | 8.78 | 10 | 29 | + |
| Compound | Matrix-Matched Calibration Curve | r | Calibration Curve in Solvent | r | Matrix Effect (%) | LOD (ng/g) | LOQ (ng/g) | RSD (%) | Recovery (%, n = 9) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day (n = 3) | Inter-Day (n = 9) | |||||||||
| BP | y = 0.116x + 0.560 | 0.999 | y = 0.121x + 0.795 | 0.999 | 96 | 0.001 | 0.003 | 12.36 | 16.79 | 81 |
| BP-1 | y = 0.167x − 0.039 | 0.999 | y = 0.175x − 0.095 | 0.999 | 96 | 0.179 | 0.537 | 3.22 | 6.36 | 82 |
| BP-2 | y = 0.093x − 0.068 | 0.999 | y = 0.044x − 0.084 | 0.998 | 210 | 0.015 | 0.045 | 4.61 | 2.60 | 71 |
| BP-3 | y= 0.226x − 0.0626 | 0.999 | y = 0.230x − 0.115 | 0.999 | 98 | 0.061 | 0.183 | 1.16 | 3.18 | 98 |
| BP-8 | y = 0.080x + 0.030 | 0.998 | y = 0.082x + 0.008 | 0.999 | 97 | 0.033 | 0.099 | 3.00 | 8.42 | 91 |
| 2-OHBP | y = 0.011x − 0.017 | 0.999 | y = 0.017x − 0.021 | 0.999 | 64 | 0.317 | 0.951 | 5.17 | 9.56 | 119 |
| 4-OHBP | y = 0.080x + 0.001 | 0.999 | y = 0.083x − 0.008 | 0.999 | 96 | 0.512 | 1.536 | 7.82 | 4.88 | 89 |
| M2BB | y = 0.716x − 0.281 | 0.999 | y = 0.52x − 0.918 | 0.998 | 137 | 0.391 | 1.173 | 4.91 | 3.79 | 114 |
| 4-MBP | y = 0.135x + 0.007 | 0.999 | y = 0.121x − 0.012 | 0.999 | 112 | 0.009 | 0.027 | 3.31 | 5.64 | 114 |
| PBZ | y = 0.089x − 0.160 | 0.999 | y = 0.044x − 0.073 | 0.999 | 204 | 0.074 | 0.222 | 11.12 | 15.08 | 95 |
| No. | Packaging Type | Chemical Nature | BP | 4-MBP | BP-3 | PBZ | 4-OHBP |
|---|---|---|---|---|---|---|---|
| 1 | Tin Can (n = 5) | Organic | 25.28 | 2.05 | <LOD | <LOD | <LOD |
| 2 | Nonorganic | 32.46 | <LOD | 0.67 | <LOD | <LOD | |
| 3 | Nonorganic | 27.39 | 1.76 | 0.58 | <LOD | <LOD | |
| 4 | Nonorganic | 25.28 | 2.05 | <LOD | <LOD | <LOD | |
| 5 | Nonorganic | 20.51 | 1.34 | 0.52 | <LOD | <LOD | |
| 6 | Aluminum Foil Bag (n = 12) | Organic | 26.00 | 1.36 | 0.49 | <LOD | <LOD |
| 7 | Organic | 30.33 | 1.89 | 0.54 | <LOD | <LOD | |
| 8 | Organic | 34.43 | 2.12 | <LOD | <LOD | <LOD | |
| 9 | Organic | 25.97 | 1.75 | <LOD | <LOD | <LOD | |
| 10 | Organic | 105.16 | 1.99 | 0.73 | <LOD | <LOD | |
| 11 | Organic | 53.88 | 2.42 | <LOD | <LOD | <LOD | |
| 12 | Nonorganic | 108.93 | <LOD | <LOD | <LOD | <LOD | |
| 13 | Nonorganic | 28.49 | 1.55 | 0.47 | <LOD | 13.98 | |
| 14 | Nonorganic | 68.79 | 2.36 | 0.68 | <LOD | <LOD | |
| 15 | Nonorganic | 19.52 | 1.57 | 0.44 | <LOD | 33.26 | |
| 16 | Nonorganic | 42.44 | 2.21 | <LOD | <LOD | <LOD | |
| 17 | Nonorganic | 43.98 | 2.22 | <LOD | <LOD | <LOD | |
| 18 | Plastic Bag (n = 8) | Organic | 32.33 | 2.09 | 0.58 | <LOD | <LOD |
| 19 | Organic | 19.13 | 1.80 | 0.69 | <LOD | <LOD | |
| 20 | Organic | 33.33 | 1.37 | 0.69 | <LOD | <LOD | |
| 21 | Organic | 70.79 | 3.31 | <LOD | <LOD | <LOD | |
| 22 | Nonorganic | 90.53 | <LOD | <LOD | 0.94 | <LOD | |
| 23 | Nonorganic | 50.99 | 1.61 | <LOD | <LOD | <LOD | |
| 24 | Nonorganic | 63.59 | 1.91 | <LOD | <LOD | <LOD | |
| 25 | Nonorganic | 64.99 | 1.87 | <LOD | <LOD | <LOD | |
| Compounds | BP | 4-MBP | BP-3 | PBZ | 4-OHBP | ||
| Detection frequency (%) | 100 | 88 | 52 | 4 | 8 | ||
| Mean (SD) | 45.8 (26.3) | 1.9 (0.4) | 0.6 (0.1) | - | 23.6 (13.6) | ||
| Geometric Mean | 39.8 | 1.9 | 0.6 | - | 21.6 | ||
| Minimum–Maximum | 19.13–108.93 | <LOD–3.3 | <LOD–0.7 | - | <LOD–33.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-R.; Huang, Y.-F.; Huang, J.-J. Identification of Benzophenone Analogs in Rice Cereal through Fast Pesticide Extraction and Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. Foods 2022, 11, 572. https://doi.org/10.3390/foods11040572
Liu X-R, Huang Y-F, Huang J-J. Identification of Benzophenone Analogs in Rice Cereal through Fast Pesticide Extraction and Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. Foods. 2022; 11(4):572. https://doi.org/10.3390/foods11040572
Chicago/Turabian StyleLiu, Xuan-Rui, Yu-Fang Huang, and Jun-Jie Huang. 2022. "Identification of Benzophenone Analogs in Rice Cereal through Fast Pesticide Extraction and Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry" Foods 11, no. 4: 572. https://doi.org/10.3390/foods11040572