Heat and Light Stability of Pumpkin-Based Carotenoids in a Photosensitive Food: A Carotenoid-Coloured Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Beverage Preparation
2.3. Storage of Samples
2.3.1. Dark Storage
2.3.2. Illuminated Storage
2.4. Ascorbic Acid Addition
2.5. Carotenoid Extraction and HPLC Analysis
2.6. Dissolved Oxygen Content Measurement
2.7. Ascorbic Acid Content Analysis
2.8. Kinetic Modelling
3. Results and Discussion
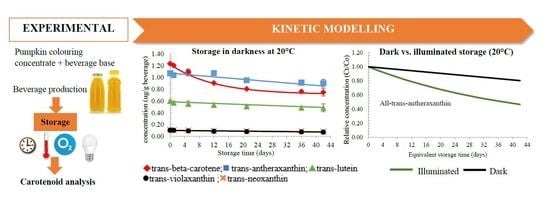
3.1. Changes in Carotenoids during Dark Storage
3.1.1. Changes in All-Trans-Carotenoid Concentrations
3.1.2. Changes in Isomer Concentrations
3.2. Changes in Carotenoids during Illuminated Storage
3.2.1. Changes in All-Trans-Carotenoid Concentrations
3.2.2. Changes in All-Trans-Carotenoid Concentrations
3.3. Predictive Modelling of Carotenoid Degradation in Both and Illuminated Conditions
3.4. Impact of Packaging and Dissolved Oxygen Content in the Samples on the Changes in All-Trans-Carotenoid Concentrations
3.5. Impact of Ascorbic Acid Addition to Changes in All-Trans-Carotenoid Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stich, E. Food Color and Coloring Food: Quality, Differentiation and Regulatory Requirements in the European Union and the United States. In Handbook on Natural Pigments in Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–27. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological Applications of Natural Colorants in Food Systems: A Review. Foods 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Natural Food Pigments and Colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Huang, W.-Y.; Li, D.-J.; Song, J.-F.; Liu, C.-Q.; Wei, Q.-Y.; Zhang, M.; Yang, Q.-M. Thermal degradation kinetics of all-trans and cis-carotenoids in a light-induced model system. Food Chem. 2018, 239, 360–368. [Google Scholar] [CrossRef]
- Achir, N.; Hadjal, T.; Madani, K.; Dornier, M.; Dhuique-Mayer, C. Carotene Reactivity in Pink Grapefruit Juice Elucidated from Model Systems and Multiresponse Modeling. J. Agric. Food Chem. 2015, 63, 3970–3979. [Google Scholar] [CrossRef]
- Hadjal, T.; Dhuique-Mayer, C.; Madani, K.; Dornier, M.; Achir, N. Thermal degradation kinetics of xanthophylls from blood orange in model and real food systems. Food Chem. 2013, 138, 2442–2450. [Google Scholar] [CrossRef]
- Manzocco, L.; Kravina, G.; Calligaris, S.; Nicoli, M.C. Shelf Life Modeling of Photosensitive Food: The Case of Colored Beverages. J. Agric. Food Chem. 2008, 56, 5158–5164. [Google Scholar] [CrossRef]
- Zepka, L.Q.; Borsarelli, C.D.; Da Silva, M.A.A.P.; Mercadante, A.Z. Thermal Degradation Kinetics of Carotenoids in a Cashew Apple Juice Model and Its Impact on the System Color. J. Agric. Food Chem. 2009, 57, 7841–7845. [Google Scholar] [CrossRef]
- Limbo, S.; Torri, L.; Piergiovanni, L. Light-Induced Changes in an Aqueous β-Carotene System Stored under Halogen and Fluorescent Lamps, Affected by Two Oxygen Partial Pressures. J. Agric. Food Chem. 2007, 55, 5238–5245. [Google Scholar] [CrossRef]
- Ghidouche, S.; Rey, B.; Michel, M.; Galaffu, N. A Rapid tool for the stability assessment of natural food colours. Food Chem. 2013, 139, 978–985. [Google Scholar] [CrossRef]
- Mesnier, X.; Gregory, C.; Fança-Berthon, P.; Boukobza, F.; Bily, A. Heat and light colour stability of beverages coloured with a natural carotene emulsion: Effect of synthetic versus natural water soluble antioxidants. Food Res. Int. 2014, 65, 149–155. [Google Scholar] [CrossRef]
- Talcott, S.T.; Percival, S.S.; Pittet-Moore, J.; Celoria, C. Phytochemical Composition and Antioxidant Stability of Fortified Yellow Passion Fruit (Passiflora edulis). J. Agric. Food Chem. 2003, 51, 935–941. [Google Scholar] [CrossRef]
- García-Alonso, F.J.; Bravo, S.; Casas, J.; Pérez-Conesa, D.; Jacob, K.; Periago, M.J. Changes in Antioxidant Compounds during the Shelf Life of Commercial Tomato Juices in Different Packaging Materials. J. Agric. Food Chem. 2009, 57, 6815–6822. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Vicario, I.M.; Heredia, F.J. Effect of ascorbic acid on deterioration of carotenoids and colour in ultrafrozen orange juice. J. Food Compos. Anal. 2009, 22, 295–302. [Google Scholar] [CrossRef]
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids: Handbook; Birkhäuser: Basel, Switzerland, 2004; ISBN 9783034878364. [Google Scholar]
- Rodriguez-Amaya, D.B. Nomenclature, Structures, and Physical and Chemical Properties. In Food Carotenoids; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–23. [Google Scholar]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; Technical Report; HarvestPlus: Washington, DC, USA, 2004. [Google Scholar]
- Vervoort, L.; Van der Plancken, I.; Grauwet, T.; Timmermans, R.A.H.; Mastwijk, H.C.; Matser, A.M.; Hendrickx, M.E.G.; Van Loey, A. Comparing equivalent thermal, high pressure and pulsed electric field processes for mild pasteurization of orange juice: Part II: Impact on specific chemical and biochemical quality parameters. Innov. Food Sci. Emerg. Technol. 2011, 12, 466–477. [Google Scholar] [CrossRef]
- Draper, N.; Richard, S.H. Applied Regression Analysis, 2nd ed.; Wiley: New York, NY, USA, 1981; ISBN 9780471029953. [Google Scholar]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes of health-related compounds throughout cold storage of tomato juice stabilized by thermal or high intensity pulsed electric field treatments. Innov. Food Sci. Emerg. Technol. 2008, 9, 272–279. [Google Scholar] [CrossRef]
- Castro-López, C.; Sánchez-Alejo, E.J.; Saucedo-Pompa, S.; Rojas, R.; Aranda-Ruiz, J.; Martínez-Avila, G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon 2016, 2, e00152. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Moreno, C.; Plaza, L.; de Ancos, B.; Cano, M.P. Vitamin C, Provitamin A Carotenoids, and Other Carotenoids in High-Pressurized Orange Juice during Refrigerated Storage. J. Agric. Food Chem. 2003, 51, 647–653. [Google Scholar] [CrossRef]
- Wibowo, S.; Vervoort, L.; Tomic, J.; Santiago, J.S.; Lemmens, L.; Panozzo, A.; Grauwet, T.; Hendrickx, M.E.; Van Loey, A. Colour and carotenoid changes of pasteurised orange juice during storage. Food Chem. 2015, 171, 330–340. [Google Scholar] [CrossRef]
- Sonar, C.R.; Paccola, C.S.; Al-Ghamdi, S.; Rasco, B.; Tang, J.; Sablani, S.S. Stability of color, β-carotene, and ascorbic acid in thermally pasteurized carrot puree to the storage temperature and gas barrier properties of selected packaging films. J. Food Process Eng. 2019, 42, e13074. [Google Scholar] [CrossRef]
- Ahmed, J.; Shivhare, U.S.; Sandhu, K.S. Thermal Degradation Kinetics of Carotenoids and Visual Color of Papaya Puree. J. Food Sci. 2002, 67, 2692–2695. [Google Scholar] [CrossRef]
- Manzocco, L.; Panozzo, A.; Calligaris, S. Accelerated Shelf Life Testing (ASLT) of Oils by Light and Temperature Exploitation. J. Am. Oil Chem. Soc. 2012, 89, 577–583. [Google Scholar] [CrossRef]
- Turcsi, E.; Nagy, V.; Deli, J. Study on the elution order of carotenoids on endcapped C18 and C30 reverse silica stationary phases. A review of the database. J. Food Compos. Anal. 2016, 47, 101–112. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, B.H. Stability of carotenoids in tomato juice during storage. Food Chem. 2005, 90, 837–846. [Google Scholar] [CrossRef]
- Chen, H.E.; Peng, H.Y.; Chen, B.H. Stability of carotenoids and vitamin A during storage of carrot juice. Food Chem. 1996, 57, 497–503. [Google Scholar] [CrossRef]
- Vásquez-Caicedo, A.L.; Schilling, S.; Carle, R.; Neidhart, S. Impact of packaging and storage conditions on colour and β-carotene retention of pasteurised mango purée. Eur. Food Res. Technol. 2007, 224, 581–590. [Google Scholar] [CrossRef]
- Mortensen, A.; Skibsted, L.H. Importance of Carotenoid Structure in Radical-Scavenging Reactions. J. Agric. Food Chem. 1997, 45, 2970–2977. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal degradation kinetics of lutein, β-carotene and β-cryptoxanthin in virgin olive oils. J. Food Compos. Anal. 2011, 24, 811–820. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Gandul-Rojas, B. Thermal Degradation Kinetics of Neoxanthin, Violaxanthin, and Antheraxanthin in Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 5180–5191. [Google Scholar] [CrossRef] [Green Version]
- Pesek, C.A.; Warthesen, J.J. Photodegradation of Carotenoids in a Vegetable Juice System. J. Food Sci. 1987, 52, 744–746. [Google Scholar] [CrossRef]
- Vidal, J.-C.; Moutounet, M. Monitoring of oxygen in the gas and liquide phases of bottles of wine at bottling and during storage. OENO One 2006, 40, 35. [Google Scholar] [CrossRef]
- Syamila, M.; Gedi, M.A.; Briars, R.; Ayed, C.; Gray, D.A. Effect of temperature, oxygen and light on the degradation of β-carotene, lutein and α-tocopherol in spray-dried spinach juice powder during storage. Food Chem. 2019, 284, 188–197. [Google Scholar] [CrossRef]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effects of industrial tomato paste processing on ascorbic acid, flavonoids and carotenoids and their stability over one-year storage. J. Sci. Food Agric. 2012, 92, 23–28. [Google Scholar] [CrossRef]
- Morais, H.; Rodrigues, P.; Ramos, C.; Forgács, E.; Cserháti, T.; Oliveira, J. Effect of Ascorbic Acid on the Stability of β-Carotene and Capsanthin in Paprika (Capsicum Annuum) Powder. Nahrung Food 2002, 46, 308–310. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, G.H.; Lee, H.S. Effects of ascorbic acid retention on juice color and pigment stability in blood orange (Citrus sinensis) juice during refrigerated storage. Food Res. Int. 2002, 35, 753–759. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef]
- Solomon, O.; Svanberg, U.; Sahlström, A. Effect of oxygen and fluorescent light on the quality of orange juice during storage at 8 °C. Food Chem. 1995, 53, 363–368. [Google Scholar] [CrossRef]



 10 °C; ■ and
10 °C; ■ and  20 °C; ▲ and
20 °C; ▲ and  35 °C; ✖ and
35 °C; ✖ and  45 °C.
45 °C.
 10 °C; ■ and
10 °C; ■ and  20 °C; ▲ and
20 °C; ▲ and  35 °C; ✖ and
35 °C; ✖ and  45 °C.
45 °C.


 ) and illuminated (
) and illuminated (  ) conditions as a function of equivalent storage time (days) at 20 °C. Carotenoid concentrations (µg/g beverage) were expressed in relative terms () where represents the carotenoid concentration at a time (equivalent storage days), is the initial carotenoid concentration at = 0 (i.e., at the start of the storage experiment, day 0).
) conditions as a function of equivalent storage time (days) at 20 °C. Carotenoid concentrations (µg/g beverage) were expressed in relative terms () where represents the carotenoid concentration at a time (equivalent storage days), is the initial carotenoid concentration at = 0 (i.e., at the start of the storage experiment, day 0).
 ) and illuminated (
) and illuminated (  ) conditions as a function of equivalent storage time (days) at 20 °C. Carotenoid concentrations (µg/g beverage) were expressed in relative terms () where represents the carotenoid concentration at a time (equivalent storage days), is the initial carotenoid concentration at = 0 (i.e., at the start of the storage experiment, day 0).
) conditions as a function of equivalent storage time (days) at 20 °C. Carotenoid concentrations (µg/g beverage) were expressed in relative terms () where represents the carotenoid concentration at a time (equivalent storage days), is the initial carotenoid concentration at = 0 (i.e., at the start of the storage experiment, day 0).

| Carotenoid | Temp. (°C) | Glass | PET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 (µg/g) | k(×10−2) a | kref (×10−2) a | Ea (kJ/mol) | r2adj | CO (µg/g) | k(×10−2) a | kref (×10−2) a | Ea (kJ/mol) | r2adj | ||
| All-trans-β-carotene Fractional conversion | 10 | 1.22 ± 0.01 | 5.41 | 7.50 ± 1.10 | 15.13 ± 3.44 | 0.98 | 1.15 ± 0.03 | 5.19 | 8.40 ± 2.0 | 22.55 ± 6.91 | 0.96 |
| 20 | 6.73 | 7.19 | |||||||||
| 35 | 9.11 | 11.29 | |||||||||
| 45 | 10.97 | 14.89 | |||||||||
| All-trans-antheraxanthin Zero order | 10 | 1.07 ± 0.01 | 0.30 | 0.64 ± 0.06 | 35.48 ± 3.53 | 0.93 | 1.04 ± 0.03 | 0.37 | 0.78 ± 0.13 | 35.23 ± 6.50 | 0.80 |
| 20 | 0.50 | 0.61 | |||||||||
| 35 | 1.02 | 1.23 | |||||||||
| 45 | 1.60 | 1.90 | |||||||||
| All-trans-lutein Zero order | 10 | 0.59 ± 0.01 | 0.16 | 0.28 ± 0.01 | 26.75 ± 3.74 | 0.87 | 0.59 ± 0.01 | 0.13 | 0.31 ± 0.08 | 41.16 ± 9.67 | 0.71 |
| 20 | 0.23 | 0.25 | |||||||||
| 35 | 0.39 | 0.52 | |||||||||
| 45 | 0.55 | 0.87 | |||||||||
| All-trans-violaxanthin Zero order | 10 | 0.10 ± 0.01 | 0.05 | 0.07 ± 0.01 | 19.48 ± 3.81 | 0.81 | 0.10 ± 0.01 | 0.07 | 0.11 ± 0.01 | 22.85 ± 4.08 | 0.81 |
| 20 | 0.06 | 0.09 | |||||||||
| 35 | 0.09 | 0.14 | |||||||||
| 45 | 0.12 | 0.19 | |||||||||
| All-trans-neoxanthin Zero order | 10 | 0.10 ± 0.01 | 0.05 | 0.07 ± 0.01 | 12.88 ± 3.31 | 0.82 | 0.10 ± 0.01 | 0.06 | 0.10 ± 0.01 | 26.20 ± 3.95 | 0.85 |
| 20 | 0.06 | 0.08 | |||||||||
| 35 | 0.08 | 0.14 | |||||||||
| 45 | 0.10 | 0.19 | |||||||||
| Carotenoid | Temp. (°C) | Glass | PET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 (µg/g) | k(×10−2) a | kref (×10−2) a | Ea (kJ/mol) | r2adj | CO (µg/g) | k(×10−2) a | kref (×10−2) a | Ea (kJ/mol) | r2adj | ||
| All-trans-β-carotene Fractional conversion | 10 | 1.19 ± 0.02 | 2.44 | 3.68 ± 0.29 | 19.25 ± 2.58 | 0.96 | 1.16 ± 0.02 | 2.54 | 3.69 ± 0.35 | 17.43 ± 3.14 | 0.96 |
| 20 | 3.22 | 3.27 | |||||||||
| 35 | 4.73 | 4.63 | |||||||||
| 45 | 6.00 | 5.73 | |||||||||
| All-trans-antheraxanthin Fractional conversion | 10 | 1.08 ± 0.02 | 2.68 | 3.79 ± 0.33 | 16.20 ± 2.87 | 0.98 | 1.06 ± 0.03 | 2.75 | 3.51 ± 0.44 | 11.50 ± 3.94 | 0.96 |
| 20 | 3.39 | 3.25 | |||||||||
| 35 | 4.68 | 4.08 | |||||||||
| 45 | 5.71 | 4.70 | |||||||||
| All-trans-lutein First order | 10 | 0.64 ± 0.01 | 1.02 | 1.21 ± 0.03 | 7.86 ± 1.21 | 0.99 | 0.67 ± 0.01 | 1.24 | 1.50 ± 0.04 | 8.36 ± 1.52 | 0.99 |
| 20 | 1.15 | 1.40 | |||||||||
| 35 | 1.35 | 1.66 | |||||||||
| 45 | 1.48 | 1.84 | |||||||||
| All-trans-violaxanthin First order | 10 | 0.10 ± 0.01 | 1.52 | 2.49 ± 0.11 | 22.94 ± 3.58 | 0.96 | 0.10 ± 0.01 | 1.79 | 3.25 ± 0.10 | 27.90 ± 3.96 | 0.98 |
| 20 | 2.12 | 2.68 | |||||||||
| 35 | 3.35 | 4.68 | |||||||||
| 45 | 4.44 | 6.60 | |||||||||
| All-trans-neoxanthin First order | 10 | 0.09 ± 0.01 | 2.08 | 3.29 ± 0.17 | 21.44 ± 3.97 | 0.97 | 0.09 ± 0.01 | 2.12 | 3.43 ± 0.17 | 22.42 ± 3.91 | 0.98 |
| 20 | 2.84 | 2.94 | |||||||||
| 35 | 4.36 | 4.60 | |||||||||
| 45 | 5.67 | 6.06 | |||||||||
| Carotenoid | Kinetic Model Order (Dark) | k (×10−2) * (Dark Storage) | Kinetic Model Order (Illuminated) | k * (Illuminated Storage) | ||
|---|---|---|---|---|---|---|
| Without Added AA | With Added AA | Without Added AA | With Added AA | |||
| All-trans-β-carotene | First-order | 1.70 ± 0.20 a | 0.39 ± 0.01 b | First-order | 1.68 ± 0.10 a | 0.23 ± 0.03 b |
| All-trans-antheraxanthin | Zero-order | 1.23 ± 0.06 a | 0.79 ± 0.11 b | First-order | 2.31 ± 0.10 a | 0.28 ± 0.05 b |
| All-trans-lutein | Zero-order | 0.52 ± 0.01 a | 0.28 ± 0.08 b | First-order | 1.34 ± 0.06 a | 0.26 ± 0.03 b |
| All-trans-violaxanthin | Zero-order | 0.14 ± 0.01 a | 0.09 ± 0.01 b | First-order | 2.06 ± 0.11 a | 0.78 ± 0.05 b |
| All-trans-neoxanthin | Zero-order | 0.14 ± 0.01 a | 0.08 ± 0.01 b | First-order | 2.75 ± 0.17 a | 1.30 ± 0.12 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atencio, S.; Verkempinck, S.H.E.; Reineke, K.; Hendrickx, M.; Van Loey, A. Heat and Light Stability of Pumpkin-Based Carotenoids in a Photosensitive Food: A Carotenoid-Coloured Beverage. Foods 2022, 11, 485. https://doi.org/10.3390/foods11030485
Atencio S, Verkempinck SHE, Reineke K, Hendrickx M, Van Loey A. Heat and Light Stability of Pumpkin-Based Carotenoids in a Photosensitive Food: A Carotenoid-Coloured Beverage. Foods. 2022; 11(3):485. https://doi.org/10.3390/foods11030485
Chicago/Turabian StyleAtencio, Sharmaine, Sarah H. E. Verkempinck, Kai Reineke, Marc Hendrickx, and Ann Van Loey. 2022. "Heat and Light Stability of Pumpkin-Based Carotenoids in a Photosensitive Food: A Carotenoid-Coloured Beverage" Foods 11, no. 3: 485. https://doi.org/10.3390/foods11030485