Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Determination of Apoptosis Rate of B16F10 Cells
2.5. Assay of Tyrosinase Activity
2.6. Measurement of Melanin Synthesis
2.7. Assay of Antioxidant Activity
2.8. Measurement of Intracellular cAMP Concentration
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of FTGML on B16F10 Cells Viability
3.2. Effects of FTGML on Apoptosis Rate of B16F10 Cells
3.3. Effects of FTGML on Intracellular Melanogenesis
3.4. Effects of FTGML on Intracellular Antioxidant Activity
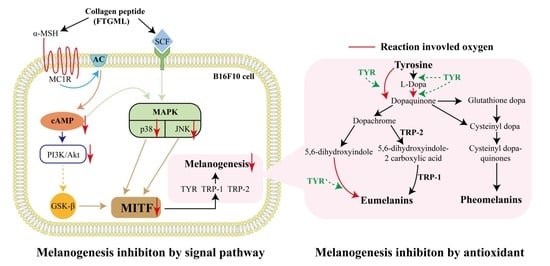
3.5. Effects of FTGML on the Melanogenesis-Related Signaling Pathway in B16F10 Melanoma Cells
3.6. Principal Component (PCA), Cluster Analyses (HCA), and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Ou-Yang, C.; Huang, Q.; Lin, M.Z.; Wang, Y.X.; Xu, K.L.; Huang, W.Y.; Pang, D.D. Antityrosinase and antioxidant properties of mung bean seed proanthocyanidins: Novel insights into the inhibitory mechanism. Food Chem. 2018, 260, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kwon, O.; Yoon, T.J.; Chung, J.H. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J. Dermatol. Sci. 2014, 75, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.G.; Choi, E.J.; Choi, Y.; Hwang, J.K. 5,7-Dimethoxyflavone induces melanogenesis in B16F10 melanoma cells through cAMP-dependent signalling. Exp. Dermatol. 2011, 20, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Gil, H.-N.; Choi, J.H.; Lim, Y.; Lee, Y.H. Agerarin inhibits α-MSH–induced TYR gene transcription via STAT3 suppression independent of CREB-MITF pathway. J. Dermatol. Sci. 2018, 91, 107–110. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Kim, Y.J.; Kim, M.O.; Kang, M.; Oh, S.W.; Nho, Y.H.; Park, S.H.; Lee, J. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem.-Biol. Interact. 2017, 273, 107–114. [Google Scholar] [CrossRef]
- Huang, H.C.; Lin, H.; Huang, M.C. The lactoferricin B-derived peptide, LfB17-34, induces melanogenesis in B16F10 cells. Int. J. Mol. Med. 2017, 39, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Oh, G.W.; Ko, S.C.; Heo, S.Y.; Nguyen, V.T.; Kim, G.; Jang, C.H.; Park, W.S.; Choi, I.W.; Qian, Z.J.; Jung, W.K. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process Biochem. 2015, 50, 1318–1326. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Hu, X.; Zhang, Y.; Pan, J.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on tyrosinase: Inhibitory interaction, conformational change and computational simulation. Food Funct. 2020, 11, 4892–4902. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, Y.S.; Lee, H.G.; Lee, J.S.; Jeon, Y.J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J.-A. Anti-melanogenic effects of epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG) and gallocatechin-3-gallate (GCG) via down-regulation of cAMP/CREB /MITF signaling pathway in B16F10 melanoma cells. Fitoterapia 2020, 145, 104634. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Otsuka, F.; Sano, A.; Tokutake, S.; Saito, M.; Kikuchi, M.; Kubota, Y. Lightening Effect on Ultraviolet-Induced Pigmentation of Guinea Pig Skin by Oral Administration of a Proanthocyanidin-Rich Extract from Grape Seeds. Pigment Cell Melanoma Res. 2010, 16, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Analysis of initial melanogenesis including tyrosinase transfer and melanosome differentiation through interrupted melanization by glutathione. J. Investig. Dermatol. 1989, 93, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumano, Y.; Sakamoto, T.; Egawa, M.; Iwai, I.; Tanaka, M.; Yamamoto, I. In vitro and in vivo prolonged biological activities of novel vitamin C derivative, 2-O-alpha-D-glucopyranosyl-L-ascorbic acid (AA-2G), in cosmetic fields. J. Nutr. Sci. Vitaminol. 1998, 44, 345–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, O.; Watabe, T.; Nakamura, K.; Kawagoshi, M.; Uotsu, N.; Chiba, T.; Yamada, M.; Yamaguchi, K.; Yamada, K.; Miyamoto, K. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, I.; Nihei, K.; Tsujimoto, K. Methyl p-coumarate, a melanin formation inhibitor in B16 mouse melanoma cells. Bioorg. Med. Chem. 2004, 12, 5349–5354. [Google Scholar] [CrossRef]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.A.F.D.; Garbelotti, M.L.; Neto, J.M.M. The application of hierarchical clusters analysis to the study of the composition of foods. Food Chem. 2006, 99, 622–629. [Google Scholar] [CrossRef]
- Cha, J.Y.; Yang, H.J.; Moon, H.I.; Cho, Y.S. Inhibitory effect and mechanism on melanogenesis from fermented herbal composition for medical or food uses. Food Res. Int. 2012, 45, 225–231. [Google Scholar] [CrossRef]
- Zhuang, Y.L.; Ma, Q.Y.; Guo, Y.; Sun, L.P. Protective effects of rambutan (Nephelium lappaceum) peel phenolics on H2O2-induced oxidative damages in HepG2 cells and D-galactose induced aging mice. Food Chem. Toxicol. 2017, 108, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.Q.; Liu, B.; Wang, Y.P.; Li, J.K.; Zhu, P.L.; Li, T.; Tse, K.W.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; et al. Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression. Cell Death Dis. 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Bowman, T.; Huang, M.; Shivers, S.; Reintgen, D.; Daud, A.; Chang, A.; Kraker, A.; Jove, R.; Yu, H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002, 21, 7001–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Bang, J.S.; Choi, Y.J.; Choung, S.-Y. Anti-melanogenic effects of oyster hydrolysate in UVB-irradiated C57BL/6J mice and B16F10 melanoma cells via downregulation of cAMP signaling pathway. J. Ethnopharmacol. 2019, 229, 137–144. [Google Scholar] [CrossRef]
- Yamamura, T.; Onishi, J.; Nishiyama, T. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch. Dermatol. Res. 2002, 294, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-González, E.; Tovar-Pérez, E.; Alcantara, L. Anti-elastase, anti-tyrosinase and antioxidant properties of a peptide fraction obtained from sorghum (Sorghum bicolor L. Moench) grain. Int. Food Res. J. 2019, 26, 1813–1822. [Google Scholar]
- Delmarmol, V.; Solano, F.; Sels, A.; Huez, G.; Libert, A.; Lejeune, F.; Ghanem, G. Glutathione Depletion Increases Tyrosinase Activity in Human-Melanoma Cells. J. Investig. Dermatol. 1993, 101, 871–874. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of α-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef]
- Wu, J.H.; Sun, B.G.; Luo, X.L.; Zhao, M.M.; Zheng, F.P.; Sun, J.Y.; Li, H.H.; Sun, X.T.; Huang, M.Q. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Chou, Y.C.; Wu, C.Y.; Chang, T.M. [8]-Gingerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem. Biophys. Res. Commun. 2013, 438, 375–381. [Google Scholar] [CrossRef] [Green Version]
- de Castro, R.J.S.; Sato, H.H. Biologically active peptides: Processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res. Int. 2015, 74, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Lorencini, M.; Brohem, C.A.; Dieamant, G.C.; Zanchin, N.I.T.; Maibach, H.I. Active ingredients against human epidermal aging. Ageing Res. Rev. 2014, 15, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Kwon, M.; Choi, J.; Kim, H.R. Sargaquinoic acid ameliorates hyperpigmentation through cAMP and ERK-mediated downregulation of MITF in α-MSH-stimulated B16F10 cells. Biomed. Pharmacother. 2018, 104, 582–589. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Su, X.R.; Liu, S.S.; Yang, S.S.; Jiang, C.Y.; Zhang, Y.; Zhang, S.C. Zebrafish phosvitin-derived peptide Pt5 inhibits melanogenesis via cAMP pathway. Fish Physiol. Biochem. 2017, 43, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.-P.; Ballotti, R.; Bertolotto, C. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 2002, 277, 33690–33697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.J.; Tsai, T.L.; Hsieh, Y.S.; Wang, C.J.; Chiou, H.L. Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch. Toxicol. 2013, 87, 1927–1937. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Sung, J.H.; Lee, S.K. Antimelanogenesis Activity of Hydrolyzed Ginseng Extract (GINST) via Inhibition of JNK Mitogen-activated Protein Kinase in B16F10 Cells. J. Food Sci. 2016, 81, H2085–H2092. [Google Scholar] [CrossRef]
- Chang, L.F.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef]
- Krens, S.F.G.; Spaink, H.P.; Snaar-Jagalska, B.E. Functions of the MAPK family in vertebrate-development. FEBS Lett. 2006, 580, 4984–4990. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The Melanogenesis Alteration Effects of Achillea millefolium L. Essential Oil and Linalyl Acetate: Involvement of Oxidative Stress and the JNK and ERK Signaling Pathways in Melanoma Cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]






| Items | Variance of Initial Eigenvalues | Extract Square Sum Load Variance | ||||
|---|---|---|---|---|---|---|
| Eigenvalue | Variance Contribution Rate/% | Accumulative Contribution Rate/% | Eigenvalue | Variance Contribution Rate/% | Accumulative Contribution Rate/% | |
| Principal component 1 | 8.714 | 87.144 | 87.144 | 5.311 | 53.110 | 53.110 |
| Principal component 2 | 0.769 | 7.692 | 94.836 | 4.173 | 41.727 | 94.836 |
| Principal component 3 | 0.325 | 3.246 | 98.083 | |||
| Principal component 4 | 0.140 | 1.401 | 99.484 | − | ||
| Principal component 5 | 0.052 | 0.516 | 100.000 | |||
| Principal component 6 | 2.834 × 10−16 | 2.834 × 10−15 | 100.000 | |||
| Principal component 7 | 4.313 × 10−17 | 4.313 × 10−16 | 100.000 | |||
| Principal component 8 | −3.498 × 10−17 | −3.498 × 10−16 | 100.000 | |||
| Principal component 9 | −1.600 × 10−16 | −1.600 × 10−15 | 100.000 | |||
| Principal component 10 | −2.691 × 10−16 | −2.691 × 10−15 | 100.000 | |||
| Items | Principal Component 1 | Principal Component 2 | ||
|---|---|---|---|---|
| Feature Vector | Load | Feature Vector | Load | |
| melanin | 0.154 | 0.778 | −0.011 | 0.562 |
| cAMP | 0.170 | 0.807 | −0.024 | 0.567 |
| tyrosinase | −0.154 | 0.529 | 0.342 | 0.824 |
| GSH | −0.146 | −0.781 | −0.001 | −0.580 |
| GSSG | −0.366 | 0.285 | 0.567 | 0.926 |
| CAT | −0.259 | −0.863 | 0.130 | −0.475 |
| Gpx | 0.238 | −0.451 | −0.436 | −0.884 |
| MDA | 0.225 | 0.845 | −0.089 | 0.513 |
| ROS | 0.147 | 0.800 | 0.005 | 0.599 |
| SOD | −0.442 | −0.884 | 0.372 | −0.185 |
| Dosage of FTGML (mg/mL) | Principal Component 1 Score (F1) | Principal Component 2 Score (F2) | Comprehensive Score (F) | Ranking |
|---|---|---|---|---|
| 0 | 0.10872 | 1.90461 | 85.25 | 6 |
| 0.1 | 1.26466 | −0.23872 | 57.20 | 5 |
| 0.2 | 0.39347 | −0.03859 | 19.29 | 4 |
| 0.4 | 0.60744 | −1.04331 | −11.27 | 3 |
| 0.8 | −1.03487 | −0.12622 | −60.23 | 2 |
| 1.6 | −1.33942 | −0.45777 | −90.24 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Sha, X.; Zhang, L.; Huang, S.; Tu, Z. Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties. Foods 2022, 11, 391. https://doi.org/10.3390/foods11030391
Hu Z, Sha X, Zhang L, Huang S, Tu Z. Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties. Foods. 2022; 11(3):391. https://doi.org/10.3390/foods11030391
Chicago/Turabian StyleHu, Zizi, Xiaomei Sha, Lu Zhang, Sheng Huang, and Zongcai Tu. 2022. "Effect of Grass Carp Scale Collagen Peptide FTGML on cAMP-PI3K/Akt and MAPK Signaling Pathways in B16F10 Melanoma Cells and Correlation between Anti-Melanin and Antioxidant Properties" Foods 11, no. 3: 391. https://doi.org/10.3390/foods11030391