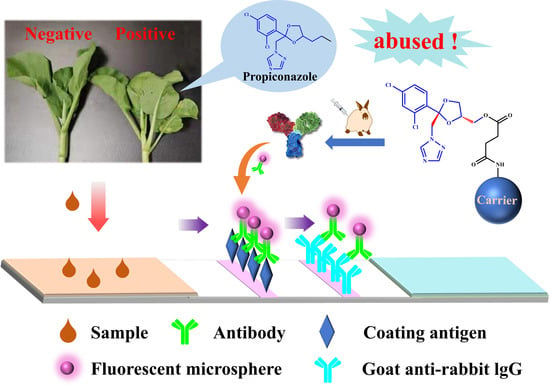
Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Apparatus
2.3. Synthesis of Hapten AZC-HS
2.4. Preparation of Immunogen and Coating Antigen
2.5. Antibody Generation
2.6. Molecular Surface Electrostatic Potential Simulation
2.7. Preparation of TRFM Labeled Antibody
2.8. Fabrication of the Lateral Flow Strip
2.9. Sample Preparation
2.10. Principle and Detection Protocol of TRFMs-LFIA
2.11. Performance of TRFMs-LFIA
3. Results and Discussion
3.1. Hapten Design and Antibody Evaluation
3.2. Characterization of TRFM Labeled Antibody
3.3. Optimization of TRFMs-LFIA
3.3.1. Activation pH
3.3.2. Antibody Dilution Buffer
3.3.3. Antibody Amount
3.3.4. Usage of Probe
3.4. Sensitivity
3.5. Specificity
3.6. Recovery
3.7. Analysis for Blind Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bai, A.; Chen, A.; Chen, W.; Luo, X.; Liu, S.; Zhang, M.; Liu, Y.; Zhang, D. Study on degradation behaviour, residue distribution, and dietary risk assessment of propiconazole in celery and onion under field application. J. Sci. Food Agric. 2021, 101, 1998–2005. [Google Scholar] [CrossRef]
- Mao, L.G.; Dong-Mei, X.U.; Tian, M.Q.; Yuan, S.K.; Fu-Gen, L.I.; Zhang, L.; Zhang, Y.N.; Jiang, H.Y. Analysis on recommended dosage of the strobilurin fungicides registered in China. Agrochemicals 2019, 58, 870–874. [Google Scholar] [CrossRef]
- Yang, Y.J. Detailed analysis of China and global propiconazole market in 2019. Pestic. Mark. News 2019, 14, 49–51. [Google Scholar]
- European Food Safety Authority (EFSA); Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; et al. Peer review of the pesticide risk assessment of the active substance propiconazole. EFSA J. 2017, 15, e04887. [Google Scholar] [CrossRef]
- Nesnow, S.; Grindstaff, R.D.; Lambert, G.; Padgett, W.T.; Bruno, M.; Ge, Y.; Chen, P.J.; Wood, C.E.; Murphy, L. Propiconazole increases reactive oxygen species levels in mouse hepatic cells in culture and in mouse liver by a cytochrome P450 enzyme mediated process. Chem.-Biol. Interact. 2011, 194, 79–89. [Google Scholar] [CrossRef]
- Souders, C.L., II; Xavier, P.; Perez-Rodriguez, V.; Ector, N.; Zhang, J.L.; Martyniuk, C.J. Sub-lethal effects of the triazole fungicide propiconazole on zebrafish (Danio rerio) development, oxidative respiration, and larval locomotor activity. Neurotoxicology Teratol. 2019, 74, 106809. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, F.; Zhou, Y.; Yan, S.; Tian, S.; Yan, J.; Meng, Z.; Bi, S.; Wang, C. Effect of propiconazole on the lipid metabolism of zebrafish embryos (Danio rerio). J. Agric. Food Chem. 2019, 67, 4623–4631. [Google Scholar] [CrossRef]
- Rossi, L. Propiconazole; Pesticide tolerances. Fed. Regist. 2012, 77, 38199–38204. [Google Scholar]
- Yang, X. The Nutritive composition and assessment of brassica parachinensis. Food Sci. Technol. 2002, 9, 74–76. [Google Scholar]
- Xiang, D.L. Vegetable farm “dwarf medicine” to cater to the concept of deformity Zecai. Ctry. Agric. Farmers B 2013, 2, 36–37. [Google Scholar]
- Huang, J.X.; Sun, L.; Qian, Y.E.; Gao, Y.W.; Wan, K.; Chen, H.C. Studies on effect of propiconazole on plant height and yield of flowering Chinese cabbage and its residue behavior. China Veg. 2019, 3, 47–52. [Google Scholar]
- Qian, Y.E.; Huang, J.; Deng, Y.; Gao, Y.; Liang, Y.; Sun, L. Residue and dietary exposure risk assessment of propiconazole and chlormequat in Chinese kale, flowering Chinese cabbage and Pak-Choi in Guangzhou. Chin. J. Trop. Crops 2017, 38, 752–757. [Google Scholar]
- Wang, C.; Wu, J.; Zhang, Y.; Wang, K.; Zhang, H. Field dissipation of trifloxystrobin and its metabolite trifloxystrobin acid in soil and apples. Environ. Monit. Assess. 2015, 187, 4100. [Google Scholar] [CrossRef] [PubMed]
- Database, E.P. EUROPA-Plants-EU Pesticides Database. 2015. Available online: http://ec.europa.eu/sanco_pesticides/public/?event=activesubstance.selection&language=EN (accessed on 6 January 2015).
- Song, S.L.; Zhu, R. Determination of chlorothalonil, propiconazole, hexaconazole, azoxystrobin and β-cypermethrin in groundwater by gas chromatogramy with elektron captured etector. Rock Miner. Anal. 2011, 30, 174–177. [Google Scholar] [CrossRef]
- Yan, W.U. Gas chromatography for determination of propiconazole residues in wolfberries. Ningxia J. Agric. For. Sci. Technol. 2018, 59, 3. Available online: http://cnki.cgl.org.cn/kcms/detail/detail.aspx?&DbCode=CJFQ&filename=NXNL201801011 (accessed on 1 November 2021).
- Mu, W.; Liu, F.; Sun, Z.; Wei, G.; Yang, L. Study on the quantitative analysis of tebuconazole, propiconazole and triadimefon by GC and HPLC. Pestic. Sci. Adm. 2005, 26, 1–3. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=a7ecf7f87380713c5f775ca4f2b8752a&site=xueshu_se (accessed on 1 November 2021).
- Zhang-Ming, C.; Shi-You, Y.; Rui, Z.; He, Z. Analysis of flusilazole·propiconazole by HPLC. Agrochemicals 2016, 55, 3. [Google Scholar] [CrossRef]
- Qiong, W.U.; Wang, M.; Daizhu, L.; Xiaochun, W.U.; Huilin, G.E.; Amp, A.; Center, T. Determination and analysis of propiconazole residues in banana and soil. Chin. J. Trop. Agric. 2019, 39, 6. [Google Scholar]
- Wang, L.Z.; Wang, D.F.; Zheng, J.C.; Wang, R.L.; Liu, Y.N.; Liang, M.; Sheng-Yu, L.U. GC-ms determination of residual fluosilazole propiconazole and difenoconazole in green pea. Phys. Test. Chem. Anal. Part B Chem. Anal. 2006, 42, 1025. [Google Scholar]
- Blondel, A.; Krings, B.; Ducat, N.; Pigeon, O. Validation of an analytical method for 1,2,4-triazole in soil using liquid chromatography coupled to electrospray tandem mass spectrometry and monitoring of propiconazole degradation in a batch study. J. Chromatogr. A 2018, 1562, 123–127. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, H.; Zheng, K.; Meng, B.; Wang, F.; Cui, Y.; Zeng, S.; Zhang, K.; Hu, D. Simultaneous determination and method validation of difenoconazole, propiconazole and pyraclostrobin in pepper and soil by LC-MS/MS in field trial samples from three provinces, China. Biomed. Chromatogr. Int. J. Devoted Res. Chromatogr. Methodol. Appl. Biosci. 2018, 32, e4052. [Google Scholar] [CrossRef] [PubMed]
- Preechakasedkit, P.; Pinwattana, K.; Dungchai, W.; Siangproh, W.; Chaicumpa, W.; Tongtawe, P.; Chailapakul, O. Development of a one-step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella typhi in human serum. Biosens. Bioelectron. 2012, 31, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Xu, L.G.; Suryoprabowo, S.; Song, S.S.; Kuang, H. Rapid detection of tulathromycin in pure milk and honey with an immunochromatographic test strip. Food Agric. Immunol. 2018, 29, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.K.; Meng, J.N.; Lei, Y.; Yang, X.X.; Yan, Y.Y.; Liu, H.H.; Lei, H.T.; Wang, T.C.; Shen, X.; Xu, Z.L. Highly selective monoclonal antibody-based lateral flow immunoassay for visual and sensitive determination of conazole fungicides propiconazole in vegetables. Food Addit. Contam. Part A. 2022, 39, 92–104. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Chen, H.; Sun, W.; Huang, Y.; Liu, F.; Wang, M.; Hua, X. Development of an indirect competitive enzyme-linked immunosorbent assay for propiconazole based on monoclonal antibody. Food Control 2022, 134, 108751. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Hu, X.; Xing, Y.; Zhang, G. Colloidal gold-based immunochromatographic strip assay for the rapid detection of diminazene in milk. Food Addit. Contam. Part A 2020, 37, 1667–1677. [Google Scholar] [CrossRef]
- Zhao, D.; He, L.; Pu, C.; Deng, A. A highly sensitive and specific polyclonal antibody-based enzyme-linked immunosorbent assay for detection of antibiotic olaquindox in animal feed samples. Anal. Bioanal. Chem. 2008, 391, 2653–2661. [Google Scholar] [CrossRef]
- Liu, J.; Song, S.S.; Wu, A.H.; Kuang, H.; Liu, L.Q.; Xiao, J.; Xu, C.L. Development of immunochromatographic strips for the detection of dicofol. Analyst 2021, 146, 2240–2247. [Google Scholar] [CrossRef]
- Fa Ng, S.; Zhang, B.; Ren, K.W.; Cao, M.M.; Shi, H.Y.; Wang, M.H. Development of a sensitive indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) based on the monoclonal antibody for the detection of the imidaclothiz residue. J. Agric. Food Chem. 2011, 59, 1594–1597. [Google Scholar] [CrossRef]
- Liang, Y.-F.; Zhou, X.-W.; Wang, F.; Shen, Y.-D.; Xiao, Z.-L.; Zhang, S.-W.; Li, Y.-J.; Wang, H. Development of a monoclonal antibody-based ELISA for the detection of Alternaria mycotoxin tenuazonic acid in food samples. Food Anal. Methods 2020, 13, 1594–1602. [Google Scholar] [CrossRef]
- Lei, H.; Rui, S.; Haughey, S.A.; Qiang, W.; Xu, Z.; Yang, J.; Shen, Y.; Hong, W.; Jiang, Y.; Sun, Y. Development of a specifically enhanced enzyme-linked immunosorbent assay for the detection of melamine in milk. Molecules 2011, 16, 5591. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, Z.; Li, P.; Qi, Z.; Wen, Z. Time-resolved fluorescent immunochromatography of aflatoxin b1 in soybean sauce: A rapid and sensitive quantitative analysis. Sensors 2016, 16, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Zhang, Y.; Liu, H.; Tao, X. A quadruple-label time-resolved fluorescence immunochromatographic assay for simultaneous quantitative determination of three mycotoxins in grains. Anal. Methods 2020, 12, 247–254. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Liu, Z.; Wang, J.; Hua, Q.; Liang, J.; Shen, X.; Xu, Z.; Lei, H.; Sun, Y. Latex microsphere immunochromatography for quantitative detection of dexamethasone in milk and pork. Food Chem. 2021, 345, 128607. [Google Scholar] [CrossRef]
- Jiang, W.; Beloglazova, N.V.; Wang, Z.; Jiang, H.; Wen, K.; Saeger, S.D.; Luo, P.; Wu, Y.; Shen, J. Development of a multiplex flow-through immunoaffinity chromatography test for the on-site screening of 14 sulfonamide and 13 quinolone residues in milk. Biosens. Bioelectron. 2015, 66, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Huang, D.; Ying, H.; Liu, F.; Xiaoyun, P.U. Evaluation of a time-resolved fluorescence immunochromatography for procalcitonin. J. Third Mil. Med. Univ. 2019, 41, 581–586. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Shen, P. Research progress on design and synthesis of hapten. Jiangsu J. Agric. Sci. 2009, 25, 1178–1182. [Google Scholar]
- Geng, J.M.; Long, Y.C.; Feng, J.; Ping, L.Y. Study on the relationship between structure and activity of the sterioisomers of golden cyclotriazole fungicides. J. Nanjing Agric. Univ. 2003, 26, 102–105. [Google Scholar]
- Gan, J.H.; Deng, W.; Jin-Ping, L.I.; Xiao-Hui, A.I. Artificial antigen synthesis and antibody preparation of doxycycline. J. Food Sci. Biotechnol. 2011, 30, 1673–1689. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-WXQG201102031.htm (accessed on 1 November 2021).
- Miao, L.A.; Hw, B.; Js, A.; Jian, J.A.; Yy, A.; Xin, L.A.; Yz, A.; Xs, A. Rapid, on-site, and sensitive detection of aflatoxin M1 in milk products by using time-resolved fluorescence microsphere test strip. Food Control 2020, 121, 107616. [Google Scholar] [CrossRef]
- Rodríguez-Cervantes, C.H.; Ramos, A.J.; Robledo-Marenco, M.L.; Sanchis, V.; Marín, S.; Girón-Pérez, M.I. Determination of aflatoxin and fumonisin levels through ELISA and HPLC, on tilapia feed in Nayarit, Mexico. Food Agric. Immunol. 2013, 24, 269–278. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Wu, X.; Wang, J.; Liu, Z.; Sun, Y.; Shen, X.; Lei, H. Rapid detection of adulteration of dehydroepiandrosterone in slimming products by competitive indirect enzyme-linked immunosorbent assay and lateral flow immunochromatography. Food Agric. Immunol. 2019, 30, 123–139. [Google Scholar] [CrossRef] [Green Version]
- Ying-Jie, X.U. Determination of six triazole pesticides residues in vegetables by SPE-GC-μECD. J. Anhui Agric. Sci. 2014, 42, 5813–5815. [Google Scholar] [CrossRef]
- Shao, Y.H. Simultaneous determination of 8 bactericide residues in soil by high performance liquid chromatography–tandem mass spectrometry. Chem. Anal. Meterage 2020, 29, 62–66. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Yao, X.; Wang, Z.; Dou, L.; Su, L.; Zhao, M.; Sun, J.; Zhang, D.; Wang, J. Developing a simple immunochromatography assay for clenbuterol with sensitivity by one-step staining. J. Agric. Food Chem. 2020, 68, 15509–15515. [Google Scholar] [CrossRef] [PubMed]





| Working Conditions | Optimal Value |
|---|---|
| Ion concentration of standard diluent | 0.2 M PB |
| Methanol content in diluent | 10% |
| Activation pH | 0.05 M MES (pH 5.5) |
| Coupling pH | 0.02 M BB (pH 8.0) |
| Antibody dilution buffer | 0.002 M BB (pH 8.0) |
| Antibody amount | 3.4 × 10−2 mg (per strip) |
| Time of coupling reaction | 45 min |
| Blocking time | 60 min |
| Immunoprobe amount | 8 μL |
| Method | Matrix | The Detection Lim (itmg/kg) | References |
|---|---|---|---|
| GC-MS | Banana | 0.02 | [19] |
| LC-MS/MS | Pepper soil | 0.005 0.0015 | [22] |
| LC-MS/MS | Soil | 0.004 | [21] |
| SPE-GC-μECD | Vegetable | 0.01 | [45] |
| GC-ECD | Groundwater | 2 | [15] |
| GC-MS | Snow peas | 0.003 | [20] |
| HPLC-MS | Soil | 0.005 | [46] |
| GC | Wolfberry | 0.006 | [16] |
| GC-ICA | Vegetable | 0.00013 | [25] |
| Ic ELISA | Vegetable | 0.00026 | [26] |
| TRFMs-LFIA | Brassica campestris | 0.00192 | This work |
| Samples | Spiked Level | Found ± SD | Recovery | CV |
|---|---|---|---|---|
| (ng/g) | (ng/g) | (%) | (%) | |
| brassica campestris | 10.0 | 8.6 ± 0.8 | 86.3 | 9.1 |
| 40.0 | 44.3 ± 5.4 | 110.7 | 12.2 | |
| 80.0 | 78.6 ± 12.3 | 98.3 | 15.6 | |
| lettuce | 10.0 | 8.7 ± 0.5 | 86.6 | 5.6 |
| 40.0 | 36.7 ± 5.1 | 91.6 | 13.9 | |
| 80.0 | 83.2 ± 8.3 | 104.0 | 9.9 | |
| romaine lettuce | 10.0 | 8.1 ± 0.7 | 81.2 | 8.8 |
| 40.0 | 31.5 ± 3.7 | 78.6 | 11.8 | |
| 80.0 | 71.1 ± 9.7 | 88.9 | 13.6 |
| Assay | TRFMs-LFIA | HPLC-MS/MS | |||
|---|---|---|---|---|---|
| Samples | Number | Test Value (Mean ± SD, ng/g) | CV (%) | Test Value (Mean ± SD, ng/g) | CV (%) |
| brassica campestris | Sample 1 | 14.2 ± 1.6 | 11.5 | 19.0 ± 1.8 | 9.4 |
| Sample 2 | 64.2 ± 5.3 | 8.2 | 71.3 ± 3.5 | 4.9 | |
| Sample 3 | 13.5 ± 1.8 | 13.4 | 9.5 ± 0.8 | 8.9 | |
| Sample 4 | 6.6 ± 0.9 | 13.8 | 4.6 ± 0.3 | 7.4 | |
| lettuce | Sample 5 | ND | - | ND | - |
| Sample 6 | ND | - | ND | - | |
| Sample 7 | ND | - | ND | - | |
| Sample 8 | ND | - | ND | - | |
| romaine lettuce | Sample 9 | ND | - | ND | - |
| Sample 10 | ND | - | ND | - | |
| Sample 11 | ND | - | ND | - | |
| Sample 12 | 8.8 ± 0.8 | 9.6 | 12.4 ± 1.3 | 10.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Shen, X.; Li, Z.; Wang, J.; Li, X.; Xu, Z.; Shen, Y.; Lei, Y.; Huang, X.; Wang, X.; et al. Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable. Foods 2022, 11, 324. https://doi.org/10.3390/foods11030324
Chen B, Shen X, Li Z, Wang J, Li X, Xu Z, Shen Y, Lei Y, Huang X, Wang X, et al. Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable. Foods. 2022; 11(3):324. https://doi.org/10.3390/foods11030324
Chicago/Turabian StyleChen, Bo, Xing Shen, Zhaodong Li, Jin Wang, Xiangmei Li, Zhenlin Xu, Yudong Shen, Yi Lei, Xinan Huang, Xu Wang, and et al. 2022. "Antibody Generation and Rapid Immunochromatography Using Time-Resolved Fluorescence Microspheres for Propiconazole: Fungicide Abused as Growth Regulator in Vegetable" Foods 11, no. 3: 324. https://doi.org/10.3390/foods11030324