Application of Pressurized Liquid Extractions to Obtain Bioactive Compounds from Tuber aestivum and Terfezia claveryi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Reagents
2.3. Pressurized Liquid Extractions
2.4. Determination of Truffles and PLE Extracts Composition
2.5. Testing of Cellular Antioxidant Activity (CAA)
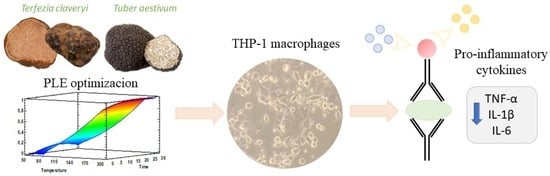
2.6. Testing of the Immunomodulatory Properties
2.7. Testing of α-Glucosidase and α-Amylase Inhibitory Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Truffles
3.2. Optimization of PLE Extraction Methods
3.2.1. Carbohydrate-Enriched Fractions
3.2.2. Protein-Enriched Fractions
3.2.3. Phenolic-Enriched Fractions
3.2.4. Sterol-Enriched Fractions
3.2.5. Multivariate Data Analysis of Extracted Compounds
3.3. Antioxidant Properties
3.4. Immunomodulatory Properties
3.5. Amylase and Glucosidase Inhibitory Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLE | pressurized liquid extraction |
| RSM | response surface methodology |
References
- Morte, A.; Kagan-Zur, V.; Navarro-Ródenas, A.; Sitrit, Y. Cultivation of Desert Truffles—A Crop Suitable for Arid and Semi-Arid Zones. Agronomy 2021, 11, 1462. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Vijay, V.; Pandey, A.K.; Semwal, A.D. Biochemical and Health Properties of Truffles. Def. Life Sci. J. 2021, 6, 251–258. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Nam, K.; Zahra, Z.; Zahra, Z.; Farooqi, M.Q.U. Potentials of truffles in nutritional and medicinal applications: A review. Fungal Biol. Biotechnol. 2020, 7, 9. [Google Scholar] [CrossRef]
- Dahham, S.S.; Al-Rawi, S.S.; Ibrahim, A.H.; Abdul Majid, A.S.; Abdul Majid, A.M.S. Antioxidant, anticancer, apoptosis properties and chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 2018, 25, 1524–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, S.A.M.; Farag, M.A.; Yosri, N.; Sabir, J.S.M.; Saeed, A.; Al-Mousawi, S.M.; Taha, W.; Musharraf, S.G.; Patel, S.; El-Seedi, H.R. Truffles: From Islamic culture to chemistry, pharmacology, and food trends in recent times. Trends Food Sci. Technol. 2019, 91, 193–218. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Amara, K.; Reis, F.S.; Barros, L.; Martins, A.; Calhelha, R.C.; Venturini, M.; Blanco, D.; Redondo, D.; Marco, P. Chemical composition and evaluation of antioxidant, antimicrobial and antiproliferative activities of Tuber and Terfezia truffles. Food Res. Int. 2020, 140, 110071. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of bioactive compounds in truffles and evaluation of pressurized liquid extractions (PLE) to obtain fractions with biological activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef]
- Harki, E.; Klaebe, A.; Talou, T.; Dargent, R. Identification and quantification of Tuber melanosporum Vitt. sterols. Steroids 1996, 61, 609–612. [Google Scholar] [CrossRef]
- Tang, Y.; Li, H.-M.; Tang, Y.-J. Comparison of sterol composition between Tuber fermentation mycelia and natural fruiting bodies. Food Chem. 2012, 132, 1207–1213. [Google Scholar] [CrossRef]
- Weete, J.D.; Abril, M.; Blackwell, M. Phylogenetic distribution of fungal sterols. PLoS ONE 2010, 5, e10899. [Google Scholar]
- Weete, J.D.; Kulifaj, M.; Montant, C.; Nes, W.R.; Sancholle, M. Distribution of sterols in fungi. II: Brassicasterol in Tuber and Terfezia species. Can. J. Microbiol. 1985, 31, 1127–1130. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Piris, A.J.; Soler-Rivas, C.; Prodanov, M. Production of a β-d-glucan-rich extract from Shiitake mushrooms (Lentinula edodes) by an extraction/microfiltration/reverse osmosis (nanofiltration) process. Innov. Food Sci. Emerg. Technol. 2019, 51, 80–90. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model. J. Funct. Foods 2014, 11, 589–597. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Aldars-García, L.; Palanisamy, M.; Jiverdeanu, R.M.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Sterol enriched fractions obtained from Agaricus bisporus fruiting bodies and by-products by compressed fluid technologies (PLE and SFE). Innov. Food Sci. Emerg. Technol. 2013, 18, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Bhotmange, D.U.; Wallenius, J.H.; Singhal, R.S.; Shamekh, S.S. Enzymatic extraction and characterization of polysaccharide from Tuber aestivum. Bioact. Carbohydr. Diet. Fibre 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Mudliyar, D.S.; Wallenius, J.H.; Bedade, D.K.; Singhal, R.S.; Madi, N.; Shamekh, S.S. Ultrasound assisted extraction of the polysaccharide from Tuber aestivum and its in vitro anti-hyperglycemic activity. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100198. [Google Scholar] [CrossRef]
- Farzaneh, P.; Khanahamadi, M.; Ehsani, M.R.; Sharifan, A. Bioactive properties of Agaricus bisporus and Terfezia claveryi proteins hydrolyzed by gastrointestinal proteases. LWT-Food Sci. Technol. 2018, 91, 322–329. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, X.H.; Li, H.M.; Wang, S.H.; Chen, T.; Yuan, Z.P.; Tang, Y.J. Isolation and characterization of polysaccharides with the antitumor activity from Tuber fruiting bodies and fermentation system. Appl. Microbiol. Biotechnol. 2014, 98, 1991–2002. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Smiderle, F.R.; Morales, D.; Govers, C.; Synytsya, A.; Wichers, H.J.; Iacomini, M.; Soler-Rivas, C. Water-soluble polysaccharide extracts from the oyster culinary-medicinal mushroom pleurotus ostreatus (Agaricomycetes) with HMGCR inhibitory activity. Int. J. Med. Mushrooms 2017, 19, 879–892. [Google Scholar] [CrossRef]
- Morales, D.; Gil-Ramirez, A.; Smiderle, F.R.; Piris, A.J.; Ruiz-Rodriguez, A.; Soler-Rivas, C. Vitamin D-enriched extracts obtained from shiitake mushrooms (Lentinula edodes) by supercritical fluid extraction and UV-irradiation. Innov. Food Sci. Emerg. Technol. 2017, 41, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC-Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Tejedor-Calvo, E.; García-Barreda, S.; Sánchez, S.; Morales, D.; Soler-Rivas, C.; Ruiz-Rodriguez, A.; Sanz, M.Á.; Garcia, A.P.; Morte, A.; Marco, P. Supercritical CO2 extraction method of aromatic compounds from truffles. LWT 2021, 150, 111954. [Google Scholar] [CrossRef]
- Palanisamy, M.; Aldars-García, L.; Gil-Ramírez, A.; Ruiz-Rodríguez, A.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Pressurized water extraction of β-glucan enriched fractions with bile acids-binding capacities obtained from edible mushrooms. Biotechnol. Prog. 2014, 30, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiderle, F.R.; Morales, D.; Gil-Ramírez, A.; de Jesus, L.I.; Gilbert-López, B.; Iacomini, M.; Soler-Rivas, C. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-D-glucans from mushrooms. Carbohydr. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Rivera, C.S.; Venturini, M.E.; Marco, P.; Oria, R.; Blanco, D. Effects of electron-beam and gamma irradiation treatments on the microbial populations, respiratory activity and sensory characteristics of Tuber melanosporum truffles packaged under modified atmospheres. Food Microbiol. 2011, 28, 1252–1260. [Google Scholar] [CrossRef]
- Morales, D.; Piris, A.J.; Ruiz-Rodriguez, A.; Prodanov, M.; Soler-Rivas, C. Extraction of bioactive compounds against cardiovascular diseases from Lentinula edodes using a sequential extraction method. Biotechnol. Prog. 2018, 34, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C. Effects of combining electron-beam or gamma irradiation treatments with further storage under modified atmospheres on the bioactive compounds of Tuber melanosporum truffles. Postharvest Biol. Technol. 2019, 155, 149–155. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Ramírez-Anguiano, A.C.; Reglero, G.; Santoyo, S. Effect of cooking, in vitro digestion and Caco-2 cells absorption on the radical scavenging activities of edible mushrooms. Int. J. Food Sci. Technol. 2009, 44, 2189–2197. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Deveci, E.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. Inhibitory activities of medicinal mushrooms on α-amylase and α-glucosidase-enzymes related to type 2 diabetes. S. Afr. J. Bot. 2021, 137, 19–23. [Google Scholar] [CrossRef]
- Malone Steverson, E.; Korus, R.A.; Admassu, W.; Heimsch, R.C. Kinetics of the amylase system of Saccharomycopsis fibuliger. Enzym. Microb. Technol. 1984, 6, 549–554. [Google Scholar] [CrossRef]
- Stojković, D.; Reis, F.S.; Ferreira, I.C.F.R.; Barros, L.; Glamočlija, J.; Ćirić, A.; Nikolić, M.; Stević, T.; Giveli, A.; Soković, M. Tirmania pinoyi: Chemical composition, in vitro antioxidant and antibacterial activities and in situ control of Staphylococcus aureus in chicken soup. Food Res. Int. 2013, 53, 56–62. [Google Scholar] [CrossRef]
- Kivrak, Ş.; Kivrak, İ. Investigation of Chemical Composition and Nutritional Value of Truffle Mushroom (Tuber nitidum Vittad.). Süleyman Demirel Üniversitesi Fen Bilim. Enstitüsü Derg. 2018, 22, 339. [Google Scholar] [CrossRef]
- Hamza, A.; Zouari, N.; Zouari, S.; Jdir, H.; Zaidi, S.; Gtari, M.; Neffati, M. Nutraceutical potential, antioxidant and antibacterial activities of Terfezia boudieri Chatin, a wild edible desert truffle from Tunisia arid zone. Arab. J. Chem. 2016, 9, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Tejedor-Calvo, E.; Morales, D.; García-Barreda, S.; Sánchez, S.; Venturini, M.E.; Blanco, D.; Soler-Rivas, C.; Marco, P. Effects of gamma irradiation on the shelf-life and bioactive compounds of Tuber aestivum truffles packaged in passive modified atmosphere. Int. J. Food Microbiol. 2020, 332, 108774. [Google Scholar] [CrossRef]
- Saltarelli, R.; Ceccaroli, P.; Cesari, P.; Barbieri, E.; Stocchi, V. Effect of storage on biochemical and microbiological parameters of edible truffle species. Food Chem. 2008, 109, 8–16. [Google Scholar] [CrossRef]
- Kıvrak, İ. Analytical Methods Applied to Assess Chemical Composition, Nutritional Value and In Vitro Bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal. Methods 2015, 8, 1279–1293. [Google Scholar] [CrossRef]
- Harki, E.; Bouya, D.; Dargent, R. Maturation-associated alterations of the biochemical characteristics of the black truffle Tuber melanosporum Vitt. Food Chem. 2006, 99, 394–400. [Google Scholar] [CrossRef]
- Parsi, Z.; Górecki, T. Determination of ergosterol as an indicator of fungal biomass in various samples using non-discriminating flash pyrolysis. J. Chromatogr. A 2006, 1130, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Sommer, K.; Vetter, W. Gas chromatography with mass spectrometry detection and characterization of 27 sterols in two truffle (Tuber) species. J. Food Compos. Anal. 2020, 94, 103650. [Google Scholar] [CrossRef]
- Pohleven, J.; Obermajer, N.; Sabotič, J.; Anžlovar, S.; Sepčić, K.; Kos, J.; Kralj, B.; Štrukelj, B.; Brzin, J. Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, M.; Stéphane, R.; Gérard, V.; Pierre-Yves, P. Pressurized water extraction of isoflavones by experimental design from soybean flour and Soybean Protein Isolate. Food Chem. 2017, 214, 9–15. [Google Scholar]
- Gil-Ramírez, A.; Clavijo, C.; Palanisamy, M.; Ruiz-Rodríguez, A.; Navarro-Rubio, M.; Pérez, M.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Study on the 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitory properties of Agaricus bisporus and extraction of bioactive fractions using pressurised solvent technologies. J. Sci. Food Agric. 2013, 93, 2789–2796. [Google Scholar] [CrossRef]
- Stanikunaite, R.; Khan, S.I.; Trappe, J.M.; Ross, S.A. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phyther. Res. 2009, 23, 575–578. [Google Scholar] [CrossRef]
- Attia, W.Y.; El-Naggar, R.E.; Bawadekji, A.; Al Ali, M. Evaluation of some in vitro anti-carcinogenic activities of polysaccharides extracted from Ascomata of the desert truffle Terfezia claveryi Chatin. J. Appl. Environ. Biol. Sci. 2018, 8, 152–159. [Google Scholar]
- Al-Laith, A. Antioxidant components and antioxidant/antiradical activities of desert truffle (Tirmania nivea) from various Middle Eastern origins. J. Food Compos. Anal. 2010, 23, 15–22. [Google Scholar] [CrossRef]
- Song, W.; Derito, C.M.; Liu, M.K.; He, X.; Dong, M.; Liu, R.H. Cellular Antioxidant Activity of Common Vegetables. J. Agric. Food Chem. 2010, 58, 6621–6629. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martínez-Tomé, M.; Jiménez, A.M.; Vera, A.M.; Honrubia, M.; Parras, P. Antioxidant Activity of Edible Fungi (Truffles and Mushrooms): Losses during Industrial Processing. J. Food Prot. 2002, 65, 1614–1622. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Clavijo, C.; Palanisamy, M.; Ruiz-Rodríguez, A.; Navarro-Rubio, M.; Pérez, M.; Marín, F.R.; Reglero, G.; Soler-Rivas, C. Screening of edible mushrooms and extraction by pressurized water (PWE) of 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitors. J. Funct. Foods 2013, 5, 244–250. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2021, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Beara, I.; Majkić, T.; Torović, L. Bioguided design of new black truffle (Tuber aestivum Vittad.) product enriched with herbs and spices. LWT 2021, 138, 110637. [Google Scholar] [CrossRef]
- Stojkovic, D.; Smiljkovic, M.; Ciric, A.; Glamoclija, J.; Van Griensven, L.; Ferreira, I.C.F.R.; Sokovic, M. An insight into antidiabetic properties of six medicinal and edible mushrooms: Inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. S. Afr. J. Bot. 2019, 120, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.Y.; Zhang, J.Y.; Chen, L.J.; Liu, X.C.; Liu, Y.; Wang, W.X.; Zhang, Y.M. Comparative evaluation of polysaccharides isolated from Astragalus, oyster mushroom, and yacon as inhibitors of α-glucosidase. Chin. J. Nat. Med. 2014, 12, 290–293. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and structural characterization of an α-glucosidase inhibitory polysaccharide from apricot (Armeniaca sibirica L. Lam.) pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H.; Hsu, C.-H.; Ng, L.-T. Inhibitory potential of fatty acids on key enzymes related to type 2 diabetes. BioFactors 2013, 39, 415–421. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Lumyong, S. Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. J. Funct. Foods 2016, 27, 352–364. [Google Scholar] [CrossRef]




| Terfezia claveryi | Tuber aestivum | |
|---|---|---|
| Carbohydrates (g/100 g) | 38.44 ± 1.35 a | 35.83 ± 0.70 b |
| β-Glucans (g/100 g) | 27.96 ± 1.55 a | 21.71 ± 0.43 b |
| Chitin (g/100 g) | 8.53 ± 0.26 a | 10.20 ± 0.63 a |
| Proteins (g/100 g) | 8.92 ± 1.05 a | 11.92 ± 0.60 a |
| TPC (mg/g) | 1.02 ± 0.07 a | 1.04 ± 0.04 a |
| Ergosterol (mg/g) | 2.30 ± 0.23 a | 2.27 ± 0.40 a |
| Brassicasterol (mg/g) | 1.40 ± 0.15 b | 1.71 ± 0.13 a |
| Ergosta7.22-dienol (mg/g) | 1.16 ± 0.02 a | 1.09 ± 0.03 a |
| Stigmasterol (mg/g) | n.d. | 0.65 ± 0.04 |
| Independent Factors | Investigated Responses | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (% Truffle) | TCH (g/100 g Extract) | β-Glucans (g/100 g Extract) | Chitin (g/100 g Extract) | Soluble Rroteins (g/100 g Extract) | TPC (mg/g Extract) | Total Sterols (mg/g Extract) | Ergosterol (mg/g Extract) | Brassicasterol (mg/g Extract) | Ergosta7.22-dienol (mg/g Extract) | Stigmasterol (mg/g Extract) | ||||||||||||||||
| Run | Temperature (°C) | Time | W | E | E:W | W | E:W | W | E:W | W | E:W | W | E:W | W | E | E:W | E | E:W | E | E:W | E | E:W | E | E:W | E | E:W |
| 1 | 50 | 5 | 39.4 | 7.6 | 9.3 | 43.15 ± 4.43 b | 40.00 ± 2.24 a | 27.92 ± 1.11 b | 19.79 ± 0.35 a | 5.97 ± 0.20 d | 3.64 ± 0.10 d | 1.87 ± 0.01 b | 1.38 ± 0.15 c | 1.84 ± 0.18 b | 0.05 ± 0.04 d | 0.24 ±0.12 d | 2.56 ± 0.18 d | n.d. | 0.47 ± 0.10 e | n.d. | 2.09 ± 0.08 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 50 | 17.5 | 44.3 | 7.5 | 9.6 | 44.44 ± 3.78 b | 44.80 ± 3.54 a | 25.20 2.53± b | 23.34 ± 2.58 a | 6.53 ± 0.09 cd | 4.80 ± 0.10 c | 1.88 ± 0.22 b | 1.57 ± 0.03 c | 2.06 ± 0.32 ab | 0.08 ± 0.02 d | 0.29 ± 0.06 d | 2.50 ± 0.42 d | n.d. | 0.50 ± 0.08 e | n.d. | 2.00 ± 0.34 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3 | 50 | 30 | 43.5 | 7.9 | 11.2 | 44.87 ± 2.61 b | 46.81 ± 3.03 a | 23.07 ± 1.57 b | 20.82 ± 0.23 a | 6.74 ± 0.06 d | 5.20 ± 0.89 c | 2.00 ± 0.03 ab | 1.34 ± 0.10 c | 2.32 ± 0.44 ab | 0.07 ± 0.14 d | 0.34 ± 0.04 d | 2.71 ± 0.39 d | n.d. | 0.93 ± 0.06 d | n.d. | 1.78 ± 0.33 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4 | 115 | 5 | 49.4 | 13.9 | 35.9 | 50.00 ± 7.53 b | 22.67 ± 5.83 c | 32.28 ± 3.17 a | 16.03 ± 0.51 b | 7.05 ± 0.14 c | 7.91 ± 0.95 a | 1.84 ± 0.06 b | 1.11 ± 0.21 d | 1.43 ± 0.19 c | 0.31 ± 0.28 c | 0.81 ± 0.03 c | 3.74 ± 0.49 c | 0.13 ± 0.03 c | 1.41 ± 0.21 c | n.d. | 2.33 ± 0.25 b | 0.13 ± 0.03 b | n.d. | n.d. | n.d. | n.d. |
| 5 | 115 | 17.5 | 49.9 | 14.1 | 37.3 | 54.30 ± 2.84 ab | 31.84 ± 2.10 b | 35.54 ± 1.16 ª | 14.58 ± 0.70 bc | 8.91 ± 0.23 b | 7.14 ± 0.37 ab | 2.18 ± 0.05 a | 2.13 ± 0.18 b | 2.26 ± 0.16 ª | 0.40 ± 0.02 c | 0.88 ± 0.05 c | 4.27 ± 0.76 bc | 0.16 ± 0.04 c | 1.58 ± 0.32 c | n.d. | 2.20 ± 0.40 b | 0.16 ± 0.04 b | 0.48 ± 0.08 b | n.d. | n.d. | n.d. |
| 6 | 115 | 17.5 | 55.5 | 15.9 | 35.1 | 59.01 ± 2.18 ª | 36.72 ± 1.88 b | 35.85 ± 1.86 ª | 15.23 ± 0.93 b | 9.51 ± 0.56 b | 8.03 ± 0.57 a | 1.90 ± 0.17 b | 1.45 ± 0.21 c | 2.03 ± 0.07 a | 0.39 ± 0.02 c | 0.96 ± 0.01 c | 4.85 ± 0.53 bc | 0.12 ± 0.04 c | 1.96 ± 0.17 bc | n.d. | 2.45 ± 0.32 b | 0.12 ± 0.04 b | 0.44 ± 0.10 b | n.d. | n.d. | n.d. |
| 7 | 115 | 17.5 | 49.9 | 14.4 | 36.3 | 62.62 ± 3.86 a | 33.04 ± 3.01 b | 38.80 ± 2.54 a | 15.12 ± 0.60 b | 9.74 ± 0.52 b | 5.93 ± 0.41 bc | 2.34 ± 0.39 a | 1.52 ± 0.13 c | 1.88 ± 0.22 bc | 0.28 ± 0.06 c | 0.85 ± 0.23 c | 5.33 ± 0.45 b | 0.40 ± 0.08 b | 2.40 ± 0.23 b | n.d. | 2.46 ± 0.15 b | 0.28 ± 0.05 a | 0.47 ± 0.07 b | 0.12 ± 0.03 b | n.d. | n.d. |
| 8 | 115 | 30 | 49.8 | 17.4 | 35.7 | 53.23 ± 2.85 b | 36.91 ± 2.93 b | 38.74 ± 2.59 a | 16.99 ±0.22 b | 9.57 ± 0.80 b | 7.65 ± 0.24 a | 2.56 ± 0.16 a | 2.33 ± 0.27 b | 2.02 ± 0.51 bc | 0.37 ± 0.07 c | 0.88 ± 0.05 c | 5.82 ± 0.63 b | 0.50 ± 0.10 b | 2.64 ± 0.25 b | n.d. | 2.58 ± 0.26 b | 0.33 ± 0.06 a | 0.59 ± 0.12 b | 0.17 ± 0.04 ab | n.d. | n.d. |
| 9 | 180 | 5 | 68.5 | 24 | 45.1 | 50.92 ± 4.01 b | 36.40 ± 1.15 b | 35.58 ± 2.18 a | 13.38 ± 0.23 c | 10.69 ± 0.53 a | 6.32 ± 0.21 b | 2.04 ± 0.25 ab | 3.29 ± 0.34 a | 2.56 ± 0.57 a | 1.12 ± 0.20 b | 1.34 ± 0.34 b | 11.36 ± 0.97 a | 0.46 ± 0.04 b | 4.70 ± 0.53 ª | n.d. | 5.38 ± 0.34 a | 0.32 ± 0.02 a | 0.47 ± 0.10 b | 0.13 ± 0.02 b | 0.81 ± 0.10 b | n.d. |
| 10 | 180 | 17.5 | 72.5 | 28.9 | 45.4 | 49.04 ± 4.74 b | 37.08 ± 1.14 b | 36.12 ± 2.75 a | 14.23 ± 0.60 bc | 10.06 ± 0.25 a | 6.75 ± 0.26 b | 1.87 ± 0.26 b | 3.64 ± 0.18 a | 2.38 ± 0.32 ab | 1.16 ± 0.68 ab | 1.60 ± 0.48 ab | 12.49 ± 0.79 a | 0.71 ± 0.16 a | 4.93 ± 0.34 a | 0.10 ±0.03 a | 5.54 ± 0.27 a | 0.42 ± 0.08 a | 1.00 ± 0.18 a | 0.20 ± 0.05 ab | 1.02 ± 0.15 ab | n.d. |
| 11 | 180 | 30 | 71.3 | 32.4 | 45.7 | 48.86 ± 2.72 b | 37.24 ± 1.30 b | 38.12 ± 2.30 a | 13.35 ± 0.72 c | 10.74 ± 0.54 a | 7.82 ± 0.58 a | 2.03 ± 0.14 ab | 2.36 ± 0.26 b | 2.66 ± 0.05 a | 1.66 ± 0.05 a | 1.92 ± 0.04 a | 12.84 ± 0.98 a | 0.76 ± 0.14 a | 5.64 ± 0.48 a | 0.15 ± 0.04 a | 5.04 ± 0.40 a | 0.40 ± 0.07 a | 1.05 ± 0.10 ª | 0.21 ± 0.03 a | 1.11 ± 0.08 a | n.d. |
| Independent Factors | Investigated Responses | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (% Truffle) | TCH (g/100 g Extract) | β-Glucans (g/100 g Extract) | Chitin (g/100 g Extract) | Soluble Proteins (g/100 g Extract) | TPC (mg/g Extract) | Total Sterols (mg/g Extract) | Ergosterol (mg/g Extract) | Brassicasterol (mg/g Extract) | Ergosta7.22-Dienol (mg/g Extract) | Stigmasterol (mg/g Extract) | 9.19ciclolanost-7-en-3-ol (mg/g Extract) | |||||||||||||||||
| Run | Temperature (°C) | Time | W | E | E:W | W | E:W | W | E:W | W | E:W | W | E:W | W | E | E:W | E | E:W | E | E:W | E | E:W | E | E:W | E | E:W | E | E:W |
| 1 | 50 | 5 | 42.03 | 2.85 | 30.87 | 20.95 ± 3.21 e | 3.87 ± 0.26 e | 3.18 ± 0.45 d | 1.79 ± 0.26 e | 5.49 ± 0.64 e | 1.45 ± 0.23 d | 0.94 ± 0.12 a | 0.90 ± 0.21 e | 1.75 ± 0.10 b | 0.02 ± 0.01 d | 0.73 ± 0.12 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 2 | 50 | 17.5 | 42.91 | 3.30 | 30.78 | 20.03 ± 2.09 e | 6.17 ± 0.87 d | 3.86 ± 0.53 d | 1.69 ± 0.27 e | 6.25 ± 0.41 e | 3.59 ± 0.32 e | 0.99 ± 0.20 a | 0.96 ± 0.12 e | 1.66 ± 0.21 b | 0.04 ± 0.02 d | 0.78 ± 0.09 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 3 | 50 | 30 | 41.41 | 3.50 | 29.36 | 24.92 ± 1.87 e | 9.12 ± 1.02 e | 4.23 ± 0.36 d | 1.78 ± 0.21 e | 6.08 ± 0.36 e | 6.01 ± 0.45 b | 1.24 ± 0.32 a | 0.58 ± 0.16 d | 1.66 ± 0.23 b | 0.03 ± 0.01 d | 0.71 ± 0.13 b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 4 | 115 | 5 | 41.92 | 12.35 | 37.98 | 22.63 ± 1.79 e | 12.35 ± 0.90 b | 4.77 ± 0.19 ed | 2.17 ± 0.34 e | 6.30 ± 0.75 e | 9.37 ± 0.67 a | 1.18 ± 0.29 a | 1.66 ± 0.15 b | 0.98 ± 0.30 e | 0.33 ± 0.05 e | 1.03 ± 0.10 b | 8.79 ± 0.57 a | n.d. | 3.61 ± 0.24 a | n.d. | 3.66 ± 0.23 a | n.d. | 1.52 ± 0.10 b | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5 | 115 | 17.5 | 43.41 | 13.01 | 37.80 | 30.41 ± 1.90 b | 13.85 ± 1.21 b | 4.12 ± 0.56 d | 1.53 ± 0.33 e | 7.96 ± 0.54 b | 10.33 ± 1.03 a | 0.92 ± 0.35 a | 0.88 ± 0.20 ed | 1.26 ± 0.18 b e | 0.40 ± 0.04 e | 0.91 ± 0.30 b | 7.84 ± 0.60 b | 0.21 ± 0.09 a | 2.87 ± 0.18 b | 0.09 ± 0.03 b | 2.96 ± 0.19 b | 0.06 ± 0.04 a | 1.84 ± 0.21 b | 0.05 ± 0.01 a | 0.09 ± 0.02 e | n.d. | 0.07 ± 0.01 e | n.d. |
| 6 | 115 | 17.5 | 45.22 | 13.37 | 38.08 | 32.72 ± 1.07 b | 12.66 ± 1.03 b | 4.65 ± 0.25 d | 2.05 ± 0.18 e | 7.84 ± 0.67 b | 9.38 ± 0.78 a | 1.11 ± 0.23 a | 1.16 ± 0.08 e | 1.54 ± 0.27 b | 0.33 ± 0.04 e | 0.96 ± 0.21 b | 7.35 ± 0.78 b | 0.34 ± 0.10 a | 2.52 ± 0.30 b | 0.15 ± 0.02 a | 2.94 ± 0.27 b | 0.14 ± 0.03 a | 1.65 ± 0.18 b | 0.05 ± 0.02 ª | 0.12 ± 0.03 e | n.d. | 0.12 ± 0.03 a b | n.d. |
| 7 | 115 | 17.5 | 44.85 | 13.52 | 37.34 | 31.96 ± 1.68 b | 13.06 ± 0.79 b | 6.48 ± 0.37 e | 2.46 ± 0.51 e | 8.80 ± 1.10 b | 10.42 ± 1.12 a | 1.04 ± 0.10 a | 1.11 ± 0.10 e | 1.45 ± 0.16 b | 0.23 ± 0.05 e | 0.90 ± 0.07 b | 9.56 ± 0.61 a | 0.33 ± 0.07 a | 2.92 ± 0.15 b | 0.15 ± 0.01 a | 3.67 ± 0.32 a | 0.13 ± 0.02 a | 2.59 ± 0.09 a | 0.06 ± 0.02 a | 0.30 ± 0.05 a | n.d. | 0.08 ± 0.02 b | n.d. |
| 8 | 115 | 30 | 44.78 | 12.52 | 39.11 | 29.63 ± 2.83 b | 12.74 ± 0.87 b | 5.16 ± 0.69 e | 3.36 ± 0.42 b | 8.65 ± 0.98 b | 8.96 ± 0.98 a | 1.01 ± 0.12 a | 1.08 ± 0.25 e | 1.06 ± 0.09 b e | 0.36 ± 0.03 e | 0.94 ± 0.13 b | 10.13 ± 0.99 a | 0.27 ± 0.12 a | 2.99 ± 0.20 b | 0.11 ± 0.03 b | 3.81 ± 0.39 a | 0.10 ± 0.04 a | 2.98 ± 0.32 a | 0.06 ± 0.01 a | 0.18 ± 0.04 b | n.d. | 0.16 ± 0.04 a | n.d. |
| 9 | 180 | 5 | 63.66 | 20.22 | 45.00 | 36.30 ± 1.08 a | 15.76 ± 1.76 b | 12.67 ± 0.89 b | 3.52 ± 0.38 b | 11.62 ± 1.01 a | 8.70 ± 0.56 a | 0.95 ± 0.09 a | 2.31 ± 0.31 a | 2.44 ± 0.21 a | 1.18 ± 0.12 a | 1.41 ± 0.09 a b | 6.45 ± 0.60 b e | 0.30 ± 0.08 a | 1.70 ± 0.16 e | 0.10 ± 0.01 b | 2.69 ± 0.25 b | 0.12 ± 0.02 a | 1.88 ± 0.15 b | 0.08 ± 0.03 a | 0.11 ± 0.02 e | n.d. | 0.07 ± 0.02 b | n.d. |
| 10 | 180 | 17.5 | 65.66 | 22.72 | 46.35 | 34.69 ± 1.12 a | 21.65 ± 1.24 a | 21.10 ± 1.09 a | 4.53 ± 0.13 a | 12.46 ± 0.79 a | 9.94 ± 0.78 a | 1.19 ± 0.23 a | 2.15 ± 0.19 a | 1.93 ± 0.30 a b | 1.04 ± 0.08 a | 1.74 ± 0.14 a | 5.90 ± 0.59 e | 0.29 ± 0.08 a | 1.76 ± 0.12 e | 0.09 ± 0.02 b | 2.23 ± 0.18 e | 0.13 ± 0.03 a | 1.69 ± 0.26 b | 0.06 ± 0.02 a | 0.17 ± 0.02 b | n.d. | 0.05 ± 0.01 e | n.d. |
| 11 | 180 | 30 | 69.27 | 22.88 | 49.47 | 35.11 ± 1.45 a | 20.74 ± 1.45 a | 22.35 ± 1.74 a | 4.49 ± 0.25 a | 12.55 ± 0.68 a | 10.24 ± 0.71 a | 1.17 ± 0.19 a | 1.22 ± 0.17 e | 2.58 ± 0.28 a | 0.78 ± 0.07 b | 1.62 ± 0.15 a | 6.03 ± 0.63 e | 0.27 ± 0.12 a | 1.84 ± 0.19 e | 0.09 ± 0.03 b | 2.34 ± 0.19 e | 0.11 ± 0.04 a | 1.63 ± 0.19 b | 0.07 ± 0.02 a | 0.13 ± 0.03 e | n.d. | 0.09 ± 0.03 ab | n.d. |
| Truffle Species | Extraction Solvent | EC50 (µg/mL) |
|---|---|---|
| T. claveryi | W | 402.96 ± 5.91 A,a |
| E:W (1:1) | 481.73 ± 9.80 B,b | |
| E | 795.58 ± 16.81 C,b | |
| T. aestivum | W | 565.95 ± 8.77 A,b |
| E:W (1:1) | 364.73 ± 6.94 A,a | |
| E | 578.71 ± 10.25 B,a |
| Truffle Species | Extraction Solvent | α-Amylase IC50 (mG/mL) | α-Glucosidase IC50 (mG/mL) |
|---|---|---|---|
| T. claveryi | W | 66.7 ± 2.59 d | 202.62 ± 3.84 f |
| E:W (1:1) | 80.73 ± 3.61 e | 1.97 ± 0.64 c | |
| E | 195.52 ± 5.74 f | 0.01 ± 0.00 a | |
| T. aestivum | W | 9.44 ± 2.64 b | 52.91 ± 2.99 e |
| E:W (1:1) | 63.24 ± 1.98 d | 49.94 ± 5.11 e | |
| E | 52.32 ± 2.36 c | 7.94 ± 0.86 d | |
| Arcabose (1 mg/mL) | 0.67 ± 0.03 a | 0.83 ± 0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor-Calvo, E.; García-Barreda, S.; Sánchez, S.; Morte, A.; Siles-Sánchez, M.d.l.N.; Soler-Rivas, C.; Santoyo, S.; Marco, P. Application of Pressurized Liquid Extractions to Obtain Bioactive Compounds from Tuber aestivum and Terfezia claveryi. Foods 2022, 11, 298. https://doi.org/10.3390/foods11030298
Tejedor-Calvo E, García-Barreda S, Sánchez S, Morte A, Siles-Sánchez MdlN, Soler-Rivas C, Santoyo S, Marco P. Application of Pressurized Liquid Extractions to Obtain Bioactive Compounds from Tuber aestivum and Terfezia claveryi. Foods. 2022; 11(3):298. https://doi.org/10.3390/foods11030298
Chicago/Turabian StyleTejedor-Calvo, Eva, Sergi García-Barreda, Sergio Sánchez, Asunción Morte, María de las Nieves Siles-Sánchez, Cristina Soler-Rivas, Susana Santoyo, and Pedro Marco. 2022. "Application of Pressurized Liquid Extractions to Obtain Bioactive Compounds from Tuber aestivum and Terfezia claveryi" Foods 11, no. 3: 298. https://doi.org/10.3390/foods11030298