Coupled Gold Nanoparticles with Aptamers Colorimetry for Detection of Amoxicillin in Human Breast Milk Based on Image Preprocessing and BP-ANN
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent
2.2. Measurements and Apparatus
2.3. Synthesis of AuNPs
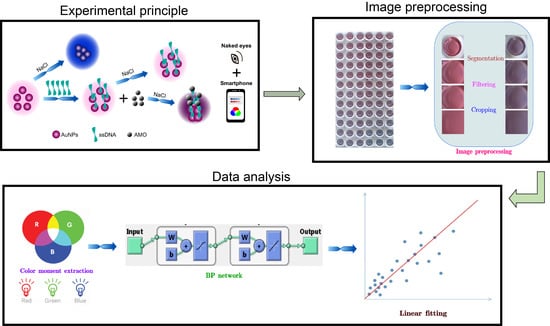
2.4. Colorimetric Detection of Amoxicillin
2.5. Image Preprocessing and Data Analysis
2.6. Preparation of Real Samples
3. Results
3.1. Design of the Experimental System
3.2. Optimization of the Conditions and Selectivity of the System
3.2.1. Optimized Reaction Reagent Concentration
3.2.2. Optimized Reaction Time
3.3. Selectivity of the Reaction System
3.4. Determination of the Amoxicillin Concentration Range
3.5. Image Preprocessing and Data Analysis
3.6. Detection of AMO in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eyrewalker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar]
- Lnnerdal, B. Bioactive Proteins in Human Milk: Mechanisms of Action. J. Pediatr. 2010, 156, S26–S30. [Google Scholar] [CrossRef] [PubMed]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes-Macías, E.; Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; González, S.; Martínez-Costa, C.; Collado, M.C. Maternal Diet Shapes the Breast Milk Microbiota Composition and Diversity: Impact of Mode of Delivery and Antibiotic Exposure. J. Nutr. 2020, 151, 330–340. [Google Scholar] [CrossRef]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2018, 143, 467–485. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; Coughlin, P.E.; Kulkarni, D.H.; Gustafsson, J.K.; Rusconi, B.; John, V.; Ndao, I.M.; Beigelman, A.; Good, M.; et al. Synchronization of mothers and offspring promotes tolerance and limits allergy. JCI Insight 2020, 5, e137943. [Google Scholar] [CrossRef]
- Nogacka, A.; Salazar, N.; Suárez, M.; Milani, C.; Arboleya, S.; Solís, G.; Fernández, N.; Alaez, L.; Hernández-Barranco, A.M.; de Los Reyes-Gavilan, C.; et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome 2017, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, P.; Curtis, N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: A systematic review. Arch. Dis. Child.—Fetal Neonatal Ed. 2019, 105, 201–208. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The effect of antibiotics on the composition of the intestinal microbiota—A systematic review. J. Infect. 2019, 79, 471–489. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, C.; Wang, C.; Song, B.; Zhou, X.; Lou, X.; He, M. Evanescent wave aptasensor for continuous and online aminoglycoside antibiotics detection based on target binding facilitated fluorescence quenching. Biosens. Bioelectron. 2017, 102, 646–651. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, T.; Beier, R.C.; Zhang, H.; Sheng, Y.; Shi, W.; Zhang, S.; Shen, J. Hapten synthesis, monoclonal antibody production and development of a competitive indirect enzyme-linked immunosorbent assay for erythromycin in milk. Food Chem. 2015, 171, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Cas, M.D.; Casagni, E.; Gambaro, V.; Cesari, E.; Roda, G. Determination of daptomycin in human plasma and breast milk by UPLC/MS-MS. J. Chromatogr. B 2019, 1116, 38–43. [Google Scholar] [CrossRef]
- Gonçalves, V.; Hazarbassanov, N.Q.; de Siqueira, A.; Florio, J.C.; Ciscato, C.H.P.; Maiorka, P.C.; Fukushima, A.R.; Spinosa, H.D.S. Development and validation of carbofuran and 3-hydroxycarbofuran analysis by high-pressure liquid chromatography with diode array detector (HPLC-DAD) for forensic Veterinary Medicine. J. Chromatogr. B 2017, 1065–1066, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, H.; Yan, L.; Li, N.; Shi, J.; Jiang, C. Recent Developments in Detection Using Noble Metal Nanoparticles. Crit. Rev. Anal. Chem. 2019, 50, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. “Gold rush” in modern science: Fabrication strategies and typical advanced applications of gold nanoparticles in sensing. Coord. Chem. Rev. 2018, 359, 1–31. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Yazdian-Robati, R.; Hedayati, N.; Dehghani, S.; Ramezani, M.; Alibolandi, M.; Saeedi, M.; Abnous, K.; Taghdisi, S.M. Application of the catalytic activity of gold nanoparticles for development of optical aptasensors. Anal. Biochem. 2021, 629, 114307. [Google Scholar] [CrossRef]
- Pham, A.; Wallace, A.; Zhang, X.; Tohl, D.; Fu, H.; Chuah, C.; Reynolds, K.; Ramsey, C.; Tang, Y. Optical-Based Biosensors and Their Portable Healthcare Devices for Detecting and Monitoring Biomarkers in Body Fluids. Diagnostics 2021, 11, 1285. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, G.; Chai, H.; Qu, L.; Zhang, X. Flexible Biosensors Based on Colorimetry, Fluorescence, and Electrochemistry for Point-of-Care Testing. Front. Bioeng. Biotechnol. 2021, 9, 753692. [Google Scholar] [CrossRef]
- Cho, S.W.; Jo, C.; Kim, Y.-H.; Park, S.K. Progress of Materials and Devices for Neuromorphic Vision Sensors. Nano-Micro Lett. 2022, 14, 203. [Google Scholar] [CrossRef]
- Liao, F.; Zhou, F.; Chai, Y. Neuromorphic vision sensors: Principle, progress and perspectives. J. Semicond. 2021, 42, 013105. [Google Scholar] [CrossRef]
- Wan, T.; Ma, S.; Liao, F.; Fan, L.; Chai, Y. Neuromorphic sensory computing. Sci. China Inf. Sci. 2021, 65, 141401. [Google Scholar] [CrossRef]
- Chai, Y. In-sensor computing for machine vision. Nature 2020, 579, 32–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuman, C.D.; Kulkarni, S.R.; Parsa, M.; Mitchell, J.P.; Date, P.; Kay, B. Opportunities for neuromorphic computing algorithms and applications. Nat. Comput. Sci. 2022, 2, 10–19. [Google Scholar] [CrossRef]
- Cleophas, T.J.; Cleophas, T.F. Artificial intelligence for diagnostic purposes: Principles, procedures and limitations. Clin. Chem. Lab. Med. 2010, 48, 159–165. [Google Scholar] [CrossRef]
- Han, F.; Huang, X.; Aheto, J.H.; Zhang, D.; Feng, F. Detection of Beef Adulterated with Pork Using a Low-Cost Electronic Nose Based on Colorimetric Sensors. Foods 2020, 9, 193. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Huang, X.; Teye, E.; Gu, H. Quantitative Analysis of Fish Microbiological Quality Using Electronic Tongue Coupled with Nonlinear Pattern Recognition Algorithms. J. Food Saf. 2015, 35, 336–344. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Zhong, J. Application of neural networks to predict the elevated temperature flow behavior of a low alloy steel. Comput. Mater. Sci. 2008, 43, 752–758. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Huang, P.-C.; Gao, N.; Li, J.-F.; Wu, F.-Y. Colorimetric detection of methionine based on anti-aggregation of gold nanoparticles in the presence of melamine. Sens. Actuators B Chem. 2018, 255, 2779–2784. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Huang, P.; Wu, F.-Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2019, 304, 125377. [Google Scholar] [CrossRef] [PubMed]





| Sample | Spiked (µM) | Determined (µM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|
| 1 | 0 | Not detected | ||
| 2 | 0.3 | 0.333 | 111.00 | 6.42 |
| 3 | 1.0 | 0.980 | 98.00 | 4.27 |
| 4 | 3.0 | 3.008 | 100.2 | 1.11 |
| Mean | 103.10 | 3.93 |
| Methodology | Linear Range | Detection Time | Apparatus | Remarks | Ref. |
|---|---|---|---|---|---|
| FQ-EWA a | 200 nM–200 µM | 10 min | Spectrofluorophotometer | Special apparatus | [10] |
| Enzyme-linked aptamer assay | 0.85–32.3 µg/L | >1 h | ELISA plate reader | Complicated operation | [11] |
| HPLC-DAD b | 6.25–100 µg/mL | >1 h | HPLC system | Time-consuming and complicated operation | [13] |
| UPLC/MS-MS | 0.12–0.32 µg/mL | >1 h | UPLC Class System | Time-consuming and complicated operation | [12] |
| Colorimetry | 0.05–3.9 µM | 10 min | Portable image acquisition device | Rapid and convenient detection | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Du, J.; Li, K.; Zhang, Z.; Xiao, P.; Yan, T.; Han, B.; Zuo, G. Coupled Gold Nanoparticles with Aptamers Colorimetry for Detection of Amoxicillin in Human Breast Milk Based on Image Preprocessing and BP-ANN. Foods 2022, 11, 4101. https://doi.org/10.3390/foods11244101
Ye Z, Du J, Li K, Zhang Z, Xiao P, Yan T, Han B, Zuo G. Coupled Gold Nanoparticles with Aptamers Colorimetry for Detection of Amoxicillin in Human Breast Milk Based on Image Preprocessing and BP-ANN. Foods. 2022; 11(24):4101. https://doi.org/10.3390/foods11244101
Chicago/Turabian StyleYe, Ziqian, Jinglong Du, Keyu Li, Zhilun Zhang, Peng Xiao, Taocui Yan, Baoru Han, and Guowei Zuo. 2022. "Coupled Gold Nanoparticles with Aptamers Colorimetry for Detection of Amoxicillin in Human Breast Milk Based on Image Preprocessing and BP-ANN" Foods 11, no. 24: 4101. https://doi.org/10.3390/foods11244101