Characterization of a Novel Polysaccharide Lyase Family 5 Alginate Lyase with PolyM Substrate Specificity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Materials
2.2. Sequence Analysis and Homology Modeling
2.3. Recombination Strains and Site-Directed Mutations
2.4. Expression and Purification of PMD and Mutants
2.5. Enzymatic Activity Assay of PMD and Mutants
2.6. Biochemical Characterization of PMD
2.7. Substrate Specificity and Kinetic Parameters
2.8. Product Analysis
2.8.1. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
2.8.2. Thin-Layer Chromatography (TLC) Analysis
2.9. Molecular Docking
2.10. Circular Dichroism (CD) Spectra
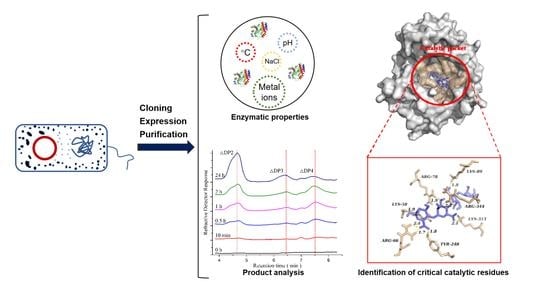
3. Results and Discussion
3.1. Cloning, Expression and Purification of Mutants
3.2. Sequence Analysis, Sequence Alignment and Homology Modeling
3.3. Enzymological Characterization of PMD
3.3.1. Effects of Temperature and pH
3.3.2. Effect of Sodium Chloride
3.3.3. Effect of Other Metal Ions
3.4. Kinetic Parameters and Substrate Specificity
3.5. Product Analysis
3.6. Molecular Docking and Potential Critical Catalytic Residues Identified
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.; Wang, P.; Zhang, Y.-Z.; Chen, X.-L. Diversity of Three-Dimensional Structures and Catalytic Mechanisms of Alginate Lyases. Appl. Environ. Microbiol. 2018, 84, e02040-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Hu, F.; Zhu, B.; Sun, Y.; Yao, Z. Biochemical Characterization and Elucidation of Action Pattern of a Novel Polysaccharide Lyase 6 Family Alginate Lyase from Marine Bacterium Flammeovirga sp. NJ-04. Mar. Drugs 2019, 17, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. Alginate Lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Qeshmi, F.I.; Homaei, A.; Khajeh, K.; Kamrani, E.; Fernandes, P. Production of a Novel Marine Pseudomonas aeruginosa Recombinant L-Asparaginase: Insight on the Structure and Biochemical Characterization. Mar. Biotechnol. 2022, 24, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Uchimura, K.; Miyazaki, M.; Nogi, Y.; Kobayashi, T.; Horikoshi, K. Cloning and Sequencing of Alginate Lyase Genes from deep-sea strains of vibrio and agarivorans and characterization of a new vibrio enzyme. Mar. Biotechnol. 2009, 12, 526–533. [Google Scholar] [CrossRef]
- Gao, S.-K.; Yin, R.; Wang, X.-C.; Jiang, H.-N.; Liu, X.-X.; Lv, W.; Ma, Y.; Zhou, Y.-X. Structure Characteristics, Biochemical Properties, and Pharmaceutical Applications of Alginate Lyases. Mar. Drugs 2021, 19, 628. [Google Scholar] [CrossRef]
- Sun, H.; Gao, L.; Xue, C.; Mao, X. Marine-polysaccharide degrading enzymes: Status and prospects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2767–2796. [Google Scholar] [CrossRef]
- Warden, A.C.; Williams, M.; Peat, T.; Seabrook, S.A.; Newman, J.; Dojchinov, G.; Haritos, V. Rational engineering of a mesohalophilic carbonic anhydrase to an extreme halotolerant biocatalyst. Nat. Commun. 2015, 6, 10278. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T.; Ko, H.-J.; Kim, N.; Kim, D.; Lee, D.; Choi, I.-G.; Woo, H.C.; Kim, M.D.; Kim, K.H. Characterization of a recombinant endo-type alginate lyase (Alg7D) from Saccharophagus degradans. Biotechnol. Lett. 2012, 34, 1087–1092. [Google Scholar] [CrossRef]
- Chen, X.-L.; Dong, S.; Xu, F.; Dong, F.; Li, P.-Y.; Zhang, X.-Y.; Zhou, B.-C.; Zhang, Y.-Z.; Xie, B.-B. Characterization of a New Cold-Adapted and Salt-Activated Polysaccharide Lyase Family 7 Alginate Lyase from Pseudoalteromonas sp. SM0524. Front. Microbiol. 2016, 7, 1120. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Koningsbruggen-Rietschel, S.; Davies, J.C.; Pressler, T.; Fischer, R.; MacGregor, G.; Donaldson, S.H.; Smerud, K.; Meland, N.; Mortensen, J.; Fosbøl, M.; et al. Inhaled dry powder alginate oligosaccharide in cystic fibrosis: A randomised, double-blind, placebo-controlled, crossover phase 2b study. ERJ Open Res. 2020, 6, 00132–02020. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Onsøyen, E.; Myrvold, R.; Howe, R.A.; Walsh, T.R.; Hill, K.E.; et al. Overcoming drug resistance with alginate oligosaccharides able to potentiate the action of selected antibiotics. Antimicrob. Agents Chemother. 2012, 56, 5134–5141. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Na, K.; Wei, J.; Zhang, L.; Guo, X. Alginate oligosaccharides: The structure-function relationships and the directional preparation for application. Carbohydr. Polym. 2022, 284, 119225. [Google Scholar] [CrossRef]
- Kraiwattanapong, J.; Tsuruga, H.; Ooi, T.; Kinoshita, S. Cloning and sequencing of a Deleya marina gene encoding for alginate lyase. Biotechnol. Lett. 1999, 21, 169–174. [Google Scholar] [CrossRef]
- Boyd, A.; Ghosh, M.; May, T.B.; Shinabarger, D.; Keogh, R.; Chakrabarty, A. Sequence of the Algl Gene of Pseudomonas Aeruginosa and Purification of its Alginate Lyase Product. Gene 1993, 131, 1–8. [Google Scholar] [CrossRef]
- Schiller, N.L.; Monday, S.R.; Boyd, C.M.; Keen, N.T.; E Ohman, D. Characterization of the Pseudomonas aeruginosa alginate lyase gene (algL): Cloning, sequencing, and expression in Escherichia coli. J. Bacteriol. 1993, 175, 4780–4789. [Google Scholar] [CrossRef] [Green Version]
- Ertesvåg, H.; Erlien, F.; Skjåk-Bræk, G.; Rehm, B.H.A.; Valla, S. Biochemical properties and substrate specificities of a recombinantly produced Azotobacter vinelandii Alginate Lyase. J. Bacteriol. 1998, 180, 3779–3784. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.-J.; Hashimoto, W.; Miyake, O.; Okamoto, M.; Mikami, B.; Murata, K. Overexpression in Escherichia coli, purification, and characterization of sphingomonas sp. A1 alginate lyases. Protein Expr. Purif. 2000, 19, 84–90. [Google Scholar] [CrossRef]
- Pecina, A.; Pascual, A.; Paneque, A. Cloning and expression of the algL gene, encoding the Azotabacter chroococcum alginate lyase: Purification and characterization of the enzyme. J. Bacteriol. 1999, 181, 1409–1414. [Google Scholar] [CrossRef]
- Preston, L.A.; Wong, T.Y.; Bender, C.L.; Schiller, N.L. Characterization of Alginate Lyase from Pseudomonas syringae pv. syringae. J. Bacteriol. 2000, 182, 6268–6271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Han, F.; Yang, Z.; Lu, X.-Z.; Yu, W.-G. A Novel Alginate Lyase with High Activity on Acetylated Alginate of Pseudomonas aeruginosa FRD1 from Pseudomonas sp. QD03. World J. Microbiol. Biotechnol. 2006, 22, 81–88. [Google Scholar] [CrossRef]
- Kam, N.; Park, Y.J.; Lee, E.Y.; Kim, H.S. Molecular identification of a polyM-specific alginate lyase from Pseudomonas sp. strain KS-408 for degradation of glycosidic linkages between two mannuronates or mannuronate and guluronate in alginate. Can. J. Microbiol. 2011, 57, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, L.C.; Berger, B. A Polysaccharide lyase from Stenotrophomonas maltophilia with a unique, pH-regulated substrate specificity. J. Biol. Chem. 2014, 289, 312–325. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.-W.; Huang, L.-S.; Tan, H.-D.; Qin, Y.-Q.; Du, Y.-G.; Yin, H. Characterization of a new endo-type polyM-specific alginate lyase from Pseudomonas sp. Biotechnol. Lett. 2014, 37, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Acharya, R. Distinct Modes of Hidden Structural Dynamics in the Functioning of an Allosteric Polysaccharide Lyase. ACS Central Sci. 2022, 8, 933–947. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput. Appl. Biosci. 1994, 10, 189–191. [Google Scholar] [CrossRef] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.; Liu, X.; Guang, C.; Zhang, W.; Mu, W. Biochemical identification of a hyperthermostable l-ribulose 3-epimerase from Labedella endophytica and its application for d-allulose bioconversion. Int. J. Biol. Macromol. 2021, 189, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Miller, G.L.; Blum, R.; Glennon, W.E.; Burton, A.L. Measurement of carboxymethylcellulase activity. Anal. Biochem. 1960, 1, 127–132. [Google Scholar] [CrossRef]
- Sun, M.; Sun, C.; Li, T.; Li, K.; Yan, S.; Yin, H. Characterization of a novel bifunctional mannuronan C-5 epimerase and alginate lyase from Pseudomonas mendocina. sp. DICP-70. Int. J. Biol. Macromol. 2020, 150, 662–670. [Google Scholar] [CrossRef]
- Hu, F.; Cao, S.; Li, Q.; Zhu, B.; Yao, Z. Construction and biochemical characterization of a novel hybrid alginate lyase with high activity by module recombination to prepare alginate oligosaccharides. Int. J. Biol. Macromol. 2021, 166, 1272–1279. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Meng, Q.; Zhou, L.; Hassanin, H.A.; Jiang, B.; Liu, Y.; Chen, J.; Zhang, T. A new role of family 32 carbohydrate binding module in alginate lyase from Vibrio natriegens SK42.001 in altering its catalytic activity, thermostability and product distribution. Food Biosci. 2021, 42, 101112. [Google Scholar] [CrossRef]
- Dong, S.; Wei, T.-D.; Chen, X.-L.; Li, C.-Y.; Wang, P.; Xie, B.-B.; Qin, Q.-L.; Zhang, X.-Y.; Pang, X.-H.; Zhou, B.-C.; et al. Molecular insight into the role of the N-terminal extension in the maturation, substrate recognition, and catalysis of a bacterial alginate lyase from polysaccharide lyase family 18. J. Biol. Chem. 2014, 289, 29558–29569. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Chen, P.; Zeng, Y.; Men, Y.; Mu, S.; Zhu, Y.; Chen, Y.; Sun, Y. The Characterization and Modification of a Novel Bifunctional and Robust Alginate Lyase Derived from Marinimicrobium sp. H1. Mar. Drugs 2019, 17, 545. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Osipiuk, J.; Chakravarthy, S.; Yuan, M.; Menzer, W.M.; Nissen, D.; Liang, P.; Raba, D.A.; Tuz, K.; Howard, A.J.; et al. Conserved residue His-257 of Vibrio cholerae flavin transferase ApbE plays a critical role in substrate binding and catalysis. J. Biol. Chem. 2019, 294, 13800–13810. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, Y.; Wang, X.; Li, H.; Ni, H.; Li, L.; Xiao, A.; Zhu, Y. Molecular cloning and characterization of AlgL17, a new exo-oligoalginate lyase from Microbulbifer sp. ALW1. Protein Expr. Purif. 2019, 161, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Bi, X.; Ren, Y.; Han, Q.; Zhou, Y.; Han, Y.; Yao, R.; Li, S. Characterization of an Alkaline Alginate Lyase with pH-Stable and Thermo-Tolerance Property. Mar. Drugs 2019, 17, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Ji, S.-Q.; Ma, X.-Q.; Lu, M.; Wang, L.-S.; Li, F.-L. Substitution of one calcium-binding amino acid strengthens substrate binding in a thermophilic alginate lyase. FEBS Lett. 2018, 592, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wen, S.; Liao, S.; Wang, Q.; Pan, S.; Zhang, R.; Lei, F.; Liao, W.; Feng, J.; Huang, S. Characterization of a bifunctional alginate lyase as a new member of the polysaccharide lyase family 17 from a marine strain BP-2. Biotechnol. Lett. 2019, 41, 1187–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.-J.; Hashimoto, W.; Miyake, O.; Murata, K.; Mikami, B. Crystal structure of alginate lyase A1-III complexed with trisaccharide product at 2.0 Å resolution. J. Mol. Biol. 2001, 307, 9–16. [Google Scholar] [CrossRef]
- Wong, S.; Eckersley, E.L.; Berger, B.; Klauda, J.B. Probing the pH Effects on Sugar Binding to a Polysaccharide Lyase. J. Phys. Chem. B 2019, 123, 7123–7136. [Google Scholar] [CrossRef]
- Pandey, S.; Mahanta, P.; Berger, B.W.; Acharya, R. Structural insights into the mechanism of pH-selective substrate specificity of the polysaccharide lyase Smlt1473. J. Biol. Chem. 2021, 297, 101014. [Google Scholar] [CrossRef]








| Name | NCBI Number | Microbial Source | Optimum | Metal Ions Dependence | Substrate Specificity | Product Analysis | Characterization Year | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | pH | ||||||||
| PMD | WP_047587958.1 | Pseudomonas mendocina | 30 | 7.0 | Yes | M-specific | DP2–3 | 2021 | This study |
| AlgL | AAA71990.1 | Pseudomonas aeruginosa | - | - | - | - | - | 1993 | [15] |
| AAG06935.1 | Pseudomonas aeruginosa | - | - | - | - | - | 1993 | [17] | |
| AAC04567.1 | Azotobacter vinelandii | - | 8.1–8.4 | Yes | M-specific | DP3–4 | 1998 | [18] | |
| algA | BAA33966.1 | Deleya marina | - | - | - | - | - | 1998 | [16] |
| A1-III | BAB03312.1 | Sphingomonas sp. A1 | 30 | 7.2 | No | M-specific | DP2–3 | 1998 | [19] |
| AlgL | CAA11481.1 | Azotobacter chroococcum | 30 | 7.5 | Yes | - | - | 1999 | [20] |
| AlgL | AAF32371.1 | Pseudomonas syringae pv. syringae | 42 | 7.0 | No | M-specific | - | 2000 | [21] |
| AAR23929.1 | Pseudomonas sp. QD03 | 37 | 7.5 | No | M-specific | DP3–5 | 2006 | [22] | |
| KS-408 | AEW23144.1 | Pseudomonas sp. strain KS-408 | 20–30 | 8.0 | No | M-specific | DP2–3 | 2011 | [23] |
| Smlt1473 | CAQ45011.1 | Stenotrophomonas maltophillia | 25 | 9.0 | - | M-specific | DP2/4/6/8 | 2013 | [24] |
| AlgA | AIY22644.1 | Pseudomonas syringae pv. | 30 | 8.0 | No | M-specific | Dp2–5 | 2014 | [25] |
| PanPL | AJE99968.1 | Pandoraea apista | - | 7.0 | - | M-specific | Dp2–3 | 2022 | [26] |
| Variants | Primers Name | Primer Sequences (5′-3′) | Tm (°C) |
|---|---|---|---|
| PMDK58A | K58A-F | 5′- TACTAGCgctTACGAGGGGTCTAACTCGGCTC-3′ | 62.5 |
| K58A-R | 5′- CCTCGTAagcGCTAGTAAACTGCAAATCGCCA-3′ | 60.2 | |
| PMDR66A | R66A-F | 5′- TAACTCGGCTgctGCAACGCTTAACCGTAAGGC-3′ | 60.3 |
| R66A-R | 5′- TTGCagcAGCCGAGTTAGACCCCTCGTACTTG-3′ | 63.4 | |
| PMDR78A | R78A-F | 5′- GAACTTCgctGAGCAGACAGCCAATATCACACG-3′ | 62.2 |
| R78A-R | 5′- TCTGCTCagcGAAGTTCTTTTCAGCCTTACGGTT-3′ | 60.8 | |
| PMDK89A | K89A-F | 5′- ACACGCCTGGAAgctGAGGCTGGGCGTATGATTACC-3′ | 61.2 |
| K89A-R | 5′- TCagcTTCCAGGCGTGTGATATTGGCTGTCTG-3′ | 62.7 | |
| PMDY248A | Y248A-F | 5′- CCCTTGCTgctCATAATTATGCCCTGCCACCA-3′ | 60.9 |
| Y248A-R | 5′- ATTATGagcAGCAAGGGCGCGCTGCGAACGAC-3′ | 63.8 | |
| PMDK313A | K313A-F | 5′-GGATTACgctTATGCCTGGCTGGCCCCATATT-3′ | 62.4 |
| K313A-R | 5′-AGGCATAagcGTAATCCTTATGCAGCTCAGCCA-3′ | 61.4 | |
| PMDR344A | R344A-F | 5′-ACAGCTTTgctCTTGGGGGCGAAGTTACTCAA-3′ | 61.8 |
| R344A-R | 5′-CCCAAGagcAAAGCTGTTGAACGGACCGCGCT-3′ | 61.4 |
| Steps | Total Protein (mg) | Total Volume (mL) | Protein Concentration (mg/mL) | Total Activity (U) | Specific Activity (U/mg) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|---|---|
| Crude enzyme | 33.02 | 15.50 | 2.13 | 126.54 | 3.83 | 100.00 | 1.00 |
| Ni-NTA | 2.48 | 2.00 | 1.24 | 111.53 | 44.97 | 88.14 | 11.74 |
| Substrate | Km (mg mL−1) | Vmax (μmol s−1) | kcat (s−1) | kcat/Km (mL mg−1 s−1) |
|---|---|---|---|---|
| Alginate | 4.03 ± 0.68 | 0.29 ± 0.02 | 232 ± 16.00 | 57.57 |
| PM | 1.32 ± 0.16 | 0.28 ± 0.01 | 224 ± 8.00 | 169.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Meng, Q.; Zhang, R.; Jiang, B.; Liu, X.; Chen, J.; Zhang, T. Characterization of a Novel Polysaccharide Lyase Family 5 Alginate Lyase with PolyM Substrate Specificity. Foods 2022, 11, 3527. https://doi.org/10.3390/foods11213527
Zhou L, Meng Q, Zhang R, Jiang B, Liu X, Chen J, Zhang T. Characterization of a Novel Polysaccharide Lyase Family 5 Alginate Lyase with PolyM Substrate Specificity. Foods. 2022; 11(21):3527. https://doi.org/10.3390/foods11213527
Chicago/Turabian StyleZhou, Licheng, Qing Meng, Ran Zhang, Bo Jiang, Xiaoyong Liu, Jingjing Chen, and Tao Zhang. 2022. "Characterization of a Novel Polysaccharide Lyase Family 5 Alginate Lyase with PolyM Substrate Specificity" Foods 11, no. 21: 3527. https://doi.org/10.3390/foods11213527