Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability
2.4. Antioxidant Enzyme Assays
2.5. Apoptosis Rate Detection
2.6. Assay for Mitochondrial Membrane Potential (MMP)
2.7. Western Blot Analysis
2.8. Inhibitor Experiments
2.9. Statistical Analysis
3. Results
3.1. PSG-1 Increased the IEC-6 Cells Viability Exposed to Acrolein
3.2. PSG-1 Attenuated Acrolein-Induced Oxidative Damage of IEC-6 Cells
3.3. Effects of PSG-1 on TJ Proteins Damaged by Acrolein in IEC-6 Cells
3.4. Effects of PSG-1 on Autophagic Proteins Damaged by Acrolein in IEC-6 Cells
3.5. Effect of PSG-1 on Acrolein-Induced Apoptosis
3.6. Effects of PSG-1 on MMP Induced by Acrolein in IEC-6 Cells
3.7. Effects of PSG-1 on Apoptotic Proteins Damaged by Acrolein in IEC-6 Cells
3.8. Effects of Autophagy Inhibitor and Apoptosis Inhibitor on the TJ in IEC-6 Cells
3.9. The Effects of Autophagy Inhibitor on IEC-6 Cell Apoptosis
3.10. The Effects of Apoptosis Inhibitor on IEC-6 Cell Autophagy
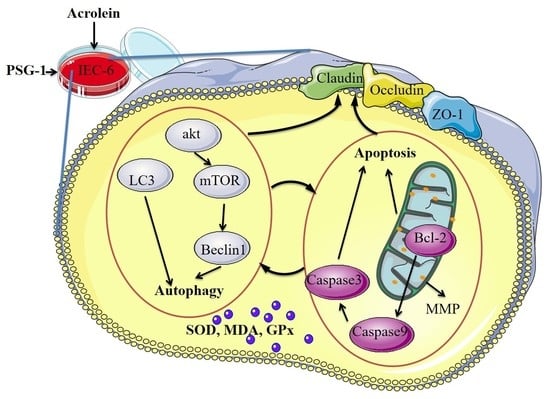
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammad Reza Zirak, S.M.; Karimani, A.; Zeinali Majid, A.; Hayes, W.; Karimi, G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food Chem. Toxicol. 2019, 129, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Lijun Chen, X.W.; Zeb, F.; Huang, Y.; An, I.; Jiang, P.; Chen, A.; Xu, C.; Feng, G. Acrolein-induced apoptosis of smooth muscle cells through NEAT1Bmal1/Clock pathway and a protection from asparagus extrac. Environ. Pollut. 2020, 258, 113735. [Google Scholar] [CrossRef]
- Jia Shi, X.-H.Z. Chemical features of the oligochitosan-glycated caseinate digest and its enhanced protection on barrier function of the acrylamide-injured IEC-6 cells. Food Chem. 2019, 290, 246–254. [Google Scholar]
- Xiaoyue Wu, L.C.; Zeb, F.; Li, C.; Jiang, P.; Chen, A.; Xu, C.; Feng, Q. Clock-Bmal1 mediates MMP9 induction in acrolein-promoted atherosclerosis associated with gut microbiota regulation. Environ. Pollut. 2019, 252, 1455–1463. [Google Scholar]
- Hou, K.Q.Y.; Hu, X.; Ding, X.; Hong, J.; Chen, Y.; Xie, J.; Nie, S.M.X. Protective effect of Ganoderma atrum polysaccharide on acrolein-induced macrophage injury via autophagy-dependent apoptosis pathway. Food Chem. Toxicol. 2019, 133, 110757. [Google Scholar] [CrossRef] [PubMed]
- Mehmet Kandemir, F.S.Y.; Kucukler, S.; Caglayan, C.; Darendelioğlu, E.; Bahaeddin Dortbudak, M. Protective effects of morin against acrylamide-induced hepatotoxicity and nephrotoxicity: A multi-biomarker approach. Food Chem. Toxicol. 2020, 138, 111190. [Google Scholar] [CrossRef]
- Isei Tanida, T.U.; Kominami, E. LC3 and autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar]
- Dan Li, L.G.; Li, M.; Luo, Y.; Xie, Y.; Luo, T.; Su, L.; Tianqiao Yong, S.C.; Jiao, C.; Su, J.; Huang, S. Polysaccharide from spore of Ganoderma lucidum ameliorates paclitaxelinduced intestinal barrier injury: Apoptosis inhibition by reversing microtubule polymerization. Biomed. Pharmacother. 2020, 130, 110539. [Google Scholar]
- Ueno, Y.; Kawamoto, Y.; Nakane, Y.; Natsume, R.; Miura, K.; Okumura, Y.; Murate, T.; Hattori, E.; Osawa, T. Oxidized perilla and linseed oils induce neuronal apoptosis by caspase-dependent and -independent pathways. Foods 2020, 9, 538. [Google Scholar] [CrossRef]
- Tu, D.G.; Chyau, C.C.; Chen, S.Y.; Chu, H.L.; Wang, S.C.; Duh, P.D. Antiproliferative Effect and mediation of apoptosis in human hepatoma HepG2 cells induced by djulis husk and its bioactive compounds. Foods 2020, 9, 1514. [Google Scholar] [CrossRef]
- Yao, F.D.J.; Cheng, F.; Yao, W.; Chen, P.; Guo, S.; Cao, Y.L.Z. Diterpene pekinenal from euphorbia pekinensis radix induced IEC-6 cells apoptosis mediated by mitochondria and death receptors. Toxicol. Vitr. 2019, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Zhao, X.-H. Activity of the peptic-tryptic caseinate digest with caseinate oligochitosan-glycation in rat intestinal epithelial (IEC-6) cells via the Wnt/beta-catenin signaling pathway. Chem. -Biol. Interact. 2020, 328, 109201. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Zhang, J.; Barve, S.S.; McClain, C.J.; Swati, J.-B. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am. J. Pathol. 2017, 187, 2686–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, K.; Chen, B.; Kang, M.; Wang, M.; Liu, K.; Wang, M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chem. 2021, 358, 129883. [Google Scholar] [CrossRef]
- Cheng, Z.Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; Wu, Z. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Che, H.Z.; Tian, Y.; Lai, P.F.H.; Xia, Y.; Wang, S.; Ai, L. Exopolysaccharide from Streptococcus thermophilus as stabilizer in fermented dairy: Binding kinetics and interactions with casein of milk. Int. J. Biol. Macromol. 2019, 140, 1018–1025. [Google Scholar] [CrossRef]
- Chen, X.Y.H.; Meng, H.; Li, W.; Li, Q.; Luo, Y.; Wang, C.; Xie, J.; Wu, L.; Zhang, X.; Wu, Z.; et al. Characteristics of the emulsion stabilized by polysaccharide conjugates alkali-extracted from green tea residue and its protective effect on catechins. Ind. Crops Prod. 2019, 140, 111611. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.-P.; Chen, Y.; Wang, Y.-X.; Xie, M.-Y. Structural characterisation of a novel bioactive polysaccharide from Ganoderma atrum. Carbohydr. Polym. 2012, 88, 1047–1054. [Google Scholar] [CrossRef]
- Zheng, B.; Xie, J.; Hong, J.; Liao, W.; Yu, Q.; Chen, Y.; Wang, Y.; Ding, X. A Ganoderma atrum polysaccharide alleviated DSS-induced ulcerative colitis by protecting the apoptosis/autophagy-regulated physical barrier and the DC-related immune barrier. Food Funct. 2020, 11, 10690–10699. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Q.; Hou, K.; Hu, X.; Wang, Y.; Chen, Y.; Xie, J.; Nie, S. Indirectly stimulation of DCs by Ganoderma atrum polysaccharide in intestinal-like Caco-2/DCs co-culture model based on RNA-seq. J. Funct. Foods 2020, 67, 103850. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Xie, J.; Zheng, B.; Chang, X.; Liu, S.; Hu, X.; Yu, Q. “Dialogue” between Caco-2 and DCs regulated by Ganoderma atrum polysaccharide in intestinal-like Caco-2/DCs co-culture model. Food Res. Int. 2021, 144, 110310. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hou, K.; Ding, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Regulatory effects of Ganoderma atrum polysaccharides on LPS-induced inflammatory macrophages model and intestinal-like Caco-2/macrophages co-culture inflammation model. Food Chem. Toxicol. 2020, 140, 111321. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, G.; Lei, A.; Yu, Q.; Xie, J.; Chen, Y. Evaluation of the protective effects of Ganoderma atrum polysaccharide on acrylamideinduced injury in small intestine tissue of rats. Food Funct. 2019, 10, 5863–5872. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, H.; Chen, Q.; Wu, X.; Yan, X.; Chen, Y.; Xie, M. Protective effects of a Ganoderma atrum polysaccharide against acrylamide induced oxidative damage via a mitochondria mediated intrinsic apoptotic pathway in IEC-6 cells. Food Funct. 2018, 9, 1133–1143. [Google Scholar] [CrossRef]
- Barraza-Garza, G.; Castillo-Michel, H.; de la Rosa, L.A.; Martinez-Martinez, A.; Cotte, M.; Alvarez-Parrilla, E. Antioxidant effect of phenolic compounds (PC) at different concentrations in IEC-6 cells: A spectroscopic analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117570. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.-M.; Lv, J.-H.; Wu, T.-C.; Zhang, Z.-P.; Liu, Y.; Chen, X.-Q.; Aweya, J.J.; Cheong, K.-L. Physicochemical properties and potential beneficial effects of porphyran from Porphyra haitanensis on intestinal epithelial cells. Carbohydr. Polym. 2020, 246, 116626. [Google Scholar] [CrossRef]
- Cai, B.; Chen, H.; Chen, D.; Chen, X.; Sun, H.; Pan, J. Composition characterization of oyster polysaccharides from Crassostrea hongkongensis and their protective effect against H2O2-induced oxidative damage in IEC-6 cells. Int. J. Biol. Macromol. 2019, 124, 246–254. [Google Scholar] [CrossRef]
- Bettaiba, J.; Droguet, M.; Magné, C.; Boulaaba, M.; Giroux-Metges, M.-A.; Ksouri, R. Tamarix gallica phenolics protect IEC-6 cells against H2O2 induced stress by restricting oxidative injuries and MAPKs signaling pathways. Biomed. Pharmacother. 2017, 89, 490–498. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Cai, W.; Qian, L. Milk Fat globule membrane ameliorates necrotizing enterocolitis in neonatal rats and suppresses lipopolysaccharide-induced inflammatory response in IEC-6 enterocytes. J. Parenter. Enter. Nutr. 2018, 43, 863–873. [Google Scholar] [CrossRef]
- Ren, B.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, Y.; Wu, C.; Jia, X.; Dong, L.; Liu, L.; Chen, Y.; Bai, Y.; Zhang, M. Rice bran phenolic extract protects against alcoholic liver injury in mice by alleviating intestinal microbiota dysbiosis, barrier dysfunction and liver inflammation mediated by endotoxin-TLR4-NF-kB pathway. J. Agric. Food Chem. 2019, 68, 1237–1247. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Liu, X.; Antonetti, D.A. Tight junctions in cell proliferation. Int. J. Mol. Sci. 2019, 20, 5972. [Google Scholar] [CrossRef] [Green Version]
- Vukelić, I.; Batičić, L.; Potočnjak, I.; Domitrović, R. Luteolin ameliorates experimental colitis in mice through ERK-mediated suppression of inflammation, apoptosis and autophagy. Food Chem. Toxicol. 2020, 145, 111680. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, Q.; Huang, J.; Luo, M.; Xiao, Y.; Mo, R.; Wang, J. Manganese (II) chloride leads to dopaminergic neurotoxicity by promoting mitophagy through BNIP3-mediated oxidative stress in SH-SY5Y cells. Cell. Mol. Biol. Lett. 2021, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, Y.H.; Xu, Q.; Liu, F.; Pang, X.N.; Wang, M.J.; Li, Q.; Li, Z.C. 4-Hydroxyderricin promotes apoptosis and cell cycle arrest through regulating PI3K/AKT/mTOR pathway in hepatocellular cells. Foods 2021, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Zhang, F.; Zhang, Y.; Thakur, K.; Naudhani, M.; Cespedes-Acuña, C.L.; Wei, Z. Stevenleaf from Gynostemma Pentaphyllum inhibits human hepatoma cell (HepG2) through cell cycle arrest and apoptotic induction. Food Sci. Hum. Wellness 2020, 9, 295–303. [Google Scholar] [CrossRef]
- Wu, Y.-j.; Zhang, F.-m.; Linhardt, R.J.; Sun, P.-l.; Zhang, A.-q. Structure, bioactivities and applications of the polysaccharides from Tremella fuciformis mushroom: A review. Int. J. Biol. Macromol. 2019, 121, 1005–1010. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Muniraj, N.; Nagalingam, A.; Walker, A.; Woo, J.; Győrffy, B.; Gabrielson, E.; Saxena, N.K.; Sharma, D. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis 2019, 40, 1110–1120. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chang, X.; Zheng, B.; Chen, Y.; Xie, J.; Shan, J.; Hu, X.; Ding, X.; Hu, X.; Yu, Q. Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells. Foods 2022, 11, 240. https://doi.org/10.3390/foods11020240
Wang Y, Chang X, Zheng B, Chen Y, Xie J, Shan J, Hu X, Ding X, Hu X, Yu Q. Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells. Foods. 2022; 11(2):240. https://doi.org/10.3390/foods11020240
Chicago/Turabian StyleWang, Yudan, Xinxin Chang, Bing Zheng, Yi Chen, Jianhua Xie, Jialuo Shan, Xiaoyi Hu, Xiaomeng Ding, Xiaobo Hu, and Qiang Yu. 2022. "Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells" Foods 11, no. 2: 240. https://doi.org/10.3390/foods11020240