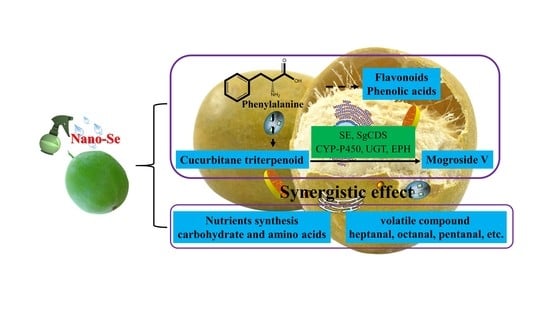
Metabolomic Analysis on the Mechanism of Nanoselenium Biofortification Improving the Siraitia grosvenorii Nutritional and Health Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiment
2.2. Mogroside V Contents and Related Key Enzyme Analyses
2.3. Flavonoids and Phenolic Acid Compounds Analyses
2.4. Carbohydrate Analyses
2.5. Amino Acid Analyses
2.6. Volatile Compounds Analyses
2.7. Widely Targeted Metabolomics Analyses
2.8. Statistical Analysis
3. Results
3.1. The Se Content and S. grosvenorii Fruit Weight
3.2. Mogroside V and the Related key Enzyme Contents in S. grosvenorii
3.3. Flavone and Phenolic Acids Contents on S. grosvenorii
3.4. The Carbohydrate Contents on S. grosvenorii
3.5. The Contents of Amino Acids on S. grosvenorii
3.6. Volatile Compounds Analyses on S. grosvenorii
3.7. Effects of Nano-Se Application on the Metabolism of S. grosvenorii Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fang, C.; Wang, Q.; Liu, X.; Xu, G. Metabolic profiling analysis of Siraitia grosvenorii revealed different characteristics of green fruit and saccharified yellow fruit. J. Pharm. Biomed. Anal. 2017, 145, 158–168. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Pan, L.C.; Zhang, L.J.; Yin, Y.; Zhu, Z.Y.; Sun, H.Q.; Liu, C.Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef]
- Qing, Z.X.; Zhao, H.; Tang, Q.; Mo, C.M.; Huang, P.; Cheng, P.; Yang, P.; Yang, X.Y.; Liu, X.B.; Zheng, Y.J.; et al. Systematic identification of flavonols, flavonol glycosides, triterpene and siraitic acid glycosides from Siraitia grosvenorii using high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry combined with a screening strategy. J. Pharm. Biomed. Anal. 2017, 138, 240–248. [Google Scholar] [CrossRef]
- Li, C.; Lin, L.-M.; Sui, F.; Wang, Z.-M.; Huo, H.-R.; Dai, L.; Jiang, T.-L. Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [CrossRef]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Lanza, M.; Reis, A.R.D. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, C.; Gao, Y.; Luo, J.; Yang, S.; Liu, S.; Zhang, R.; Zou, N. Exogenous salicylic acid mitigates the accumulation of some pesticides in cucumber seedlings under different cultivation methods. Ecotoxicol. Environ. Saf. 2020, 198, 110680. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, J.; Wu, J.; Zhang, L.; Wang, J.; Zhang, B.; Wang-Pruski, G. Alleviating effect of silicon on melon seed germination under autotoxicity stress. Ecotoxicol. Environ. Saf. 2020, 188, 109901. [Google Scholar] [CrossRef]
- Eskandari, S.; Hofte, H.; Zhang, T. Foliar manganese spray induces the resistance of cucumber to Colletotrichum lagenarium. J. Plant Physiol. 2020, 246–247, 153129. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Pilon-Smits, E.A.; Zhao, F.J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.F.; Zhang, Z.; Gao, Y.; Zhao, J. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol. Environ. Saf. 2020, 189, 109955. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zhang, J.; An, Q.; Wu, Y.; Li, J.Q.; Pan, C. Nanoselenium Foliar Applications Enhance the Nutrient Quality of Pepper by Activating the Capsaicinoid Synthetic Pathway. J. Agric. Food Chem. 2020, 68, 9888–9895. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalipour, M.; Esmaielpour, B.; Behnamian, M.; Gohari, G.; Giglou, M.T.; Vachova, P.; Rastogi, A.; Brestic, M.; Skalicky, M. Chitosan-Selenium Nanoparticle (Cs-Se NP) Foliar Spray Alleviates Salt Stress in Bitter Melon. Nanomaterials 2021, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Nakayama, Y.; Matsuda, F.; Uchikata, T.; Kobayashi, S.; Matsubara, A.; Fukusaki, E. Current metabolomics: Practical applications. J. Biosci. Bioeng. 2013, 115, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Siddique, K.H.M.; Liu, K. Cropping systems in agriculture and their impact on soil health—A review. Glob. Ecol. Conserv. 2020, 23, e01118. [Google Scholar] [CrossRef]
- Wu, F.; Ding, Y.; Nie, Y.; Wang, X.J.; An, Y.Q.; Roessner, U.; Walker, R.; Du, B.; Bai, J.G. Plant metabolomics integrated with transcriptomics and rhizospheric bacterial community indicates the mitigation effects of Klebsiella oxytoca P620 on p-hydroxybenzoic acid stress in cucumber. J. Hazard. Mater. 2021, 415, 125756. [Google Scholar] [CrossRef]
- Cao, X.; Liu, M.; Hu, Y.; Xue, Q.; Yao, F.; Sun, J.; Sun, L.; Liu, Y. Systemic characteristics of biomarkers and differential metabolites of raw and ripened pu-erh teas by chemical methods combined with a UPLC-QQQ-MS-based metabolomic approach. LWT 2021, 136, 110316. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Z.; Li, K.; Li, K.; Liu, L.; Zhang, W.; Xu, J.; Tu, X.; Du, L.; Zhang, H. Novel edible coating based on shellac and tannic acid for prolonging postharvest shelf life and improving overall quality of mango. Food Chem. 2021, 354, 129510. [Google Scholar] [CrossRef]
- Shi, J.; Wu, H.; Xiong, M.; Chen, Y.; Chen, J.; Zhou, B.; Wang, H.; Li, L.; Fu, X.; Bie, Z.; et al. Comparative analysis of volatile compounds in thirty nine melon cultivars by headspace solid-phase microextraction and gas chromatography-mass spectrometry. Food Chem. 2020, 316, 126342. [Google Scholar] [CrossRef]
- Li, D.; An, Q.; Wu, Y.; Li, J.-Q.; Pan, C. Foliar Application of Selenium Nanoparticles on Celery Stimulates Several Nutrient Component Levels by Regulating the α-Linolenic Acid Pathway. ACS Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- Huang, K.-L.; Tian, J.; Wang, H.; Fu, Y.-F.; Li, Y.; Zheng, Y.; Li, X.-B. Fatty acid export protein BnFAX6 functions in lipid synthesis and axillary bud growth in Brassica napus. Plant Physiol. 2021, 186, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Huang, M.T.; Ho, C.T. Anti-inflammatory activities of mogrosides from Momordica grosvenori in murine macrophages and a murine ear edema model. J. Agric. Food Chem. 2011, 59, 7474–7481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Zhao, L.; Zhou, G.; Li, X. Regulating the gut microbiota and SCFAs in the faeces of T2DM rats should be one of antidiabetic mechanisms of mogrosides in the fruits of Siraitia grosvenorii. J. Ethnopharmacol. 2021, 274, 114033. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Hao, J.; Xie, H.; Shimizu, K.; Li, R.; Zhang, C. Natural product mogrol attenuates bleomycin-induced pulmonary fibrosis development through promoting AMPK activation. J. Funct. Foods 2021, 77, 104280. [Google Scholar] [CrossRef]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Environ. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, X.; Yuan, X.; Wu, Q.; Chen, S.; Zou, Y.; Ma, F.; Li, C. Exogenous dopamine improves apple fruit quality via increasing flavonoids and soluble sugar contents. Sci. Hortic. 2021, 280, 109903. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic acid treatment mitigates chilling injury in peach fruit by regulation of sucrose metabolism and soluble sugar content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, L.; Li, P.; Sun, T.; Wang, C.; Li, F.; Chen, Y.; Du, B.; Yang, Z. Influence of Plant Growth Retardants on Quality of Codonopsis Radix. Molecules 2017, 22, 1655. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdan, T.; Portu, J.; Lopez, R.; Santamaria, P. Effect of methyl jasmonate application to grapevine leaves on grape amino acid content. Food Chem. 2016, 203, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, M.; Gao, C.; Fu, F.-F.; Wang, T.; El-Kassaby, Y.A.; Wang, G. Amino acid metabolism reprogramming in response to changing growth environment in Ginkgo biloba leaves. LWT 2021, 144, 111276. [Google Scholar] [CrossRef]
- Liu, K.; Li, S.; Han, J.; Zeng, X.; Ling, M.; Mao, J.; Li, Y.; Jiang, J. Effect of selenium on tea (Camellia sinensis) under low temperature: Changes in physiological and biochemical responses and quality. Environ. Exp. Bot. 2021, 188, 104475. [Google Scholar] [CrossRef]
- Wei, N.; Wang, M.; Adams, S.J.; Yu, P.; Avula, B.; Wang, Y.H.; Pan, K.; Wang, Y.; Khan, I.A. Comparative study and quality evaluation regarding morphology characters, volatile constituents, and triglycerides in seeds of five species used in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2021, 194, 113801. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Song, J.; Shao, Y.; Yan, Y.; Li, X.; Peng, J.; Guo, L. Characterization of volatile profiles of three colored quinoas based on GC-IMS and PCA. LWT 2021, 146, 111292. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef]
- Li, D.; Zhou, C.; Zou, N.; Wu, Y.; Zhang, J.; An, Q.; Li, J.Q.; Pan, C. Nanoselenium foliar application enhances biosynthesis of tea leaves in metabolic cycles and associated responsive pathways. Environ. Pollut. 2021, 273, 116503. [Google Scholar] [CrossRef]
- Zhou, C.; Li, D.; Shi, X.; Zhang, J.; An, Q.; Wu, Y.; Kang, L.; Li, J.Q.; Pan, C. Nanoselenium Enhanced Wheat Resistance to Aphids by Regulating Biosynthesis of DIMBOA and Volatile Components. J. Agric. Food Chem. 2021, 69, 14103–14114. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, J.; Wu, Y.; Cheng, H.; Pang, Q.; Xiao, Y.; Li, D.; Pan, C. Metabolomic Analysis on the Mechanism of Nanoselenium Biofortification Improving the Siraitia grosvenorii Nutritional and Health Value. Foods 2022, 11, 3019. https://doi.org/10.3390/foods11193019
Zhou C, Zhang J, Wu Y, Cheng H, Pang Q, Xiao Y, Li D, Pan C. Metabolomic Analysis on the Mechanism of Nanoselenium Biofortification Improving the Siraitia grosvenorii Nutritional and Health Value. Foods. 2022; 11(19):3019. https://doi.org/10.3390/foods11193019
Chicago/Turabian StyleZhou, Chunran, Jingbang Zhang, Yangliu Wu, Haiyan Cheng, Qiuling Pang, Yuanhui Xiao, Dong Li, and Canping Pan. 2022. "Metabolomic Analysis on the Mechanism of Nanoselenium Biofortification Improving the Siraitia grosvenorii Nutritional and Health Value" Foods 11, no. 19: 3019. https://doi.org/10.3390/foods11193019