Effect of Solid Fat Content in Fat Droplets on Creamy Mouthfeel of Acid Milk Gels
Abstract
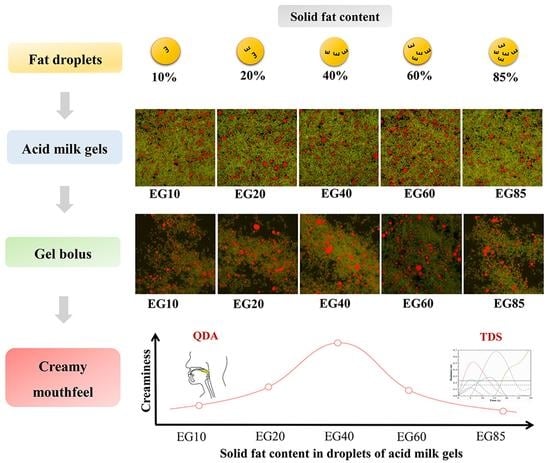
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Blended Milk Fats with Different Solid Fat Content Values
2.2.1. Preparation of the Blended Milk Fats
2.2.2. Determination of Solid Fat Content
2.2.3. Crystal Morphology
2.2.4. Thermodynamic Properties
2.3. Preparation and Characterization of Acid Milk Gels
2.3.1. Preparation of Acid Milk Gels
2.3.2. Simulated Oral Processing
2.3.3. Characterization of Gel Bolus
Particle Size Distribution
Observation of the Microstructure
Apparent Viscosity
Tribological Properties
2.3.4. Sensory Evaluation of Acid Milk Gels
Quantitative Descriptive Analysis
Temporal Dominance of Sensations Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Crystallization Behavior of Blended Milk Fats
3.1.1. Solid Fat Content
3.1.2. Crystal Morphology
3.1.3. Thermodynamic Properties
3.2. The Partial Coalescence of Fat Droplets in Gel Bolus
3.2.1. The Particle Size of Fat Droplets
3.2.2. Confocal Laser Scanning Images
3.3. The Mechanical Properties of Acid Milk Gels during Simulated Oral Processing
3.3.1. Apparent Viscosity
3.3.2. Tribological Properties
3.4. Sensory Characterization
3.4.1. QDA of Sensory Characterization
3.4.2. Temporal Dominance of Sensations Curves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickinson, E. On the road to understanding and control of creamy mouthfeel in food colloids. Food Hydrocoll. 2018, 77, 372–385. [Google Scholar] [CrossRef]
- Wijk, R.; Terpstra, M.; Janssen, A.M.; Prinz, J.F. Perceived creaminess of semi-solid foods. Trends Food Sci. Technol. 2006, 17, 412–422. [Google Scholar] [CrossRef]
- Dresselhuis, D.M.; Hoog, E.; Stuart, M.; Vingerhoeds, M.H.; Aken, G. The occurrence of in-mouth coalescence of emulsion droplets in relation to perception of fat. Food Hydrocoll. 2008, 22, 1170–1183. [Google Scholar] [CrossRef]
- Chen, J. Food oral processing: Some important underpinning principles of eating and sensory perception. Food Struct. 2014, 1, 91–105. [Google Scholar] [CrossRef]
- Sarkar, A.; Ye, A.; Singh, H. Oral processing of emulsion systems from a colloidal perspective. Food Funct. 2016, 8, 511–521. [Google Scholar] [CrossRef]
- Benjamins, J.; Vingerhoeds, M.H.; Zoet, F.D.; Hoog, E.; Aken, G. Partial coalescence as a tool to control sensory perception of emulsions. Food Hydrocoll. 2009, 23, 102–115. [Google Scholar] [CrossRef]
- Petrut, R.F.; Danthine, S.; Blecker, C. Assessment of partial coalescence in whippable oil-in-water food emulsions. Adv. Colloid Interface Sci. 2016, 229, 25–33. [Google Scholar] [CrossRef]
- Boode, K.; Walstra, P. Partial coalescence in oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 1993, 81, 121–137. [Google Scholar] [CrossRef]
- Liu, K.; Stieger, M.; van der Linden, E.; van de Velde, F. Fat droplet characteristics affect rheological, tribological and sensory properties of food gels. Food Hydrocoll. 2015, 44, 244–259. [Google Scholar] [CrossRef]
- Fredrick, E.; Walstra, P.; Dewettinck, K. Factors governing partial coalescence in oil-in-water emulsions. Adv. Colloid Interface Sci. 2010, 153, 30–42. [Google Scholar]
- Schlich, P. Temporal Dominance of Sensations (TDS): A new deal for temporal sensory analysis. Curr. Opin. Food Sci. 2017, 15, 38–42. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, H.; Shen, Q.; Liu, C.; Luo, J. A novel approach for modulating the spatial distribution of fat globules in acid milk gel and its effect on the perception of fat-related attributes. Food Res. Int. 2020, 140, 109990. [Google Scholar] [CrossRef] [PubMed]
- GB.T 31743 2015; Chinese Standard Animal and Vegetable Fats and Oils—Determination of Solid Fat Content by Pulsed NMR—Direct Method. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2015.
- Bayard, M.; Leal-Calderon, F.; Cansell, M. Free fatty acids and their esters modulate isothermal crystallization of anhydrous milk fat. Food Chem. 2017, 218, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.; Narine, S.S.; Marangoni, A.G. Effect of cooling rate on the structure and mechanical properties of milk fat and lard. Food Res. Int. 2002, 35, 971–981. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Chang, Y.; Wang, P.; Li, Y.; Zhang, H.; Luo, J. Formation and structural properties of acid-induced casein–agar double networks: Role of gelation sequence. Food Hydrocoll. 2018, 85, 291–298. [Google Scholar] [CrossRef]
- Godoi, F.C.; Bhandari, B.R.; Prakash, S. Tribo-rheology and sensory analysis of a dairy semi-solid. Food Hydrocoll. 2017, 70, 240–250. [Google Scholar]
- Truong, T.; Morgan, G.P.; Bansal, N.; Palmer, M.; Bhandari, B. Crystal structures and morphologies of fractionated milk fat in nanoemulsions. Food Chem. 2015, 171, 157–167. [Google Scholar] [CrossRef]
- Cichero, J.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P.; et al. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: Foundations of a global initiative. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 280–291. [Google Scholar] [CrossRef]
- Mu, S.; Liu, L.; Liu, H.; Shen, Q.; Luo, J. Characterization of the relationship between olfactory perception and the release of aroma compounds before and after simulated oral processing. J. Dairy Sci. 2021, 104, 2855–2865. [Google Scholar]
- Luo, J.; Wang, Y.; Guo, H.; Ren, F. Effects of size and stability of native fat globules on the formation of milk gel induced by rennet. J. Food Sci. 2017, 82, 670–678. [Google Scholar] [CrossRef]
- De Lavergne, M.D.; van Delft, M.; van de Velde, F.; van Boekel, M.A.; Stieger, M. Dynamic texture perception and oral processing of semi-solid food gels: Part 1: Comparison between QDA, progressive profiling and TDS. Food Hydrocoll. 2015, 43, 207–217. [Google Scholar] [CrossRef]
- Tournier, C.; Martin, C.; Guichard, E.; Issanchou, S.; Sulmont-Rossé, C. Contribution to the understanding of consumers’ creaminess concept: A sensory and a verbal approach. Int. Dairy J. 2007, 17, 555–564. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Z.; Cao, C.; Liu, Y. The partial coalescence behavior of oil-in-water emulsions: Comparison between refrigerated and room temperature storage. Food Chem. 2019, 300, 125219. [Google Scholar] [CrossRef] [PubMed]
- Goibier, L.; Lecomte, S.; Leal-Calderon, F.; Faure, C. The effect of surfactant crystallization on partial coalescence in O/W emulsions. J. Colloid Interface Sci. 2017, 500, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, N.; Andersen, U.; Wiking, L. The effect of cooling rate and rapeseed oil addition on the melting behaviour, texture and microstructure of anhydrous milk fat. Int. Dairy J. 2012, 25, 73–79. [Google Scholar] [CrossRef]
- Engelen, L.; de Wijk, R.A.; Prinz, J.F.; Janssen, A.M.; Weenen, H.; Bosman, F. The effect of oral and product temperature on the perception of flavor and texture attributes of semi-solids. Appetite 2003, 41, 273–281. [Google Scholar] [CrossRef]
- Liu, K.; Stieger, M.; Erik, V.; Fred, V. Effect of microparticulated whey protein on sensory properties of liquid and semi-solid model foods. Food Hydrocoll. 2016, 60, 186–198. [Google Scholar] [CrossRef]
- Wang, C.; Lou, X.; Wang, J. Fatty Acid Composition and Fat Stability of Raw Milk and Pasteurized Milk from Laoshan Goats. J. Agric. Sci. 2016, 8, 149–158. [Google Scholar] [CrossRef]
- Chojnicka-Paszun, A.; Doussinault, S.; Jongh, H. Sensorial analysis of polysaccharide–gelled protein particle dispersions in relation to lubrication and viscosity properties. Food Res. Int. 2014, 56, 199–210. [Google Scholar] [CrossRef]
- Sonne, A.; Busch-Stockfisch, M.; Weiss, J.; Hinrichs, J. Improved mapping of in-mouth creaminess of semi-solid dairy products by combining rheology, particle size, and tribology data. LWT—Food Sci. Technol. 2014, 59, 342–347. [Google Scholar] [CrossRef]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian-Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Oliver, L.; Scholten, E.; Aken, G. Effect of fat hardness on large deformation rheology of acid milk gels. Food Hydrocoll. 2015, 43, 299–310. [Google Scholar] [CrossRef]
- Selway, N.; Stokes, J.R. Soft materials deformation, flow, and lubrication between compliant substrates: Impact on flow behavior, mouthfeel, stability, and flavor. Annu. Rev. Food Sci. Technol. 2014, 5, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Shewan, H.M.; Pradal, C.; Stokes, J.R. Tribology and its growing use toward the study of food oral processing and sensory perception. J. Texture Stud. 2019, 51, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Bhandari, B.; Prakash, S. Tribological method to measure lubricating properties of dairy products. J. Food Eng. 2016, 168, 27–34. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Engelen, L.; de Wijk, R.A. Oral processing and texture perception. Food Oral Process. Fundam. Eat. Sens. Percept. 2012, 8, 157–176. [Google Scholar]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. Sequential aspects of cream cheese texture perception using temporal dominance of sensations (TDS) tool and its relation with flow and lubrication behaviour. Food Res. Int. 2018, 120, 586–594. [Google Scholar] [CrossRef]
- Thomas, A.; Chambault, M.; Dreyfuss, L.; Gilbert, C.; Hegyi, A.; Henneberg, S.; Knippertz, A.; Kostyra, E.; Kremer, S.; Silva, A. Measuring temporal liking simultaneously to Temporal Dominance of Sensations in several intakes. An application to Gouda cheeses in 6 Europeans countries. Food Res. Int. 2017, 99, 426–434. [Google Scholar] [CrossRef]






| Components | F10 | F20 | F40 | F60 | F85 |
|---|---|---|---|---|---|
| Anhydrous milk fat (%) | 30 | 30 | 50 | 50 | 10 |
| Glyceryl stearate (%) | 0 | 20 | 25 | 40 | 90 |
| Oleic acid glyceride (%) | 70 | 50 | 25 | 10 | 0 |
| Descriptor | Definition | Reference |
|---|---|---|
| Graininess | Dairy products containing grains, like particles in the mouth, which can be large and small. | Milk = 0; Oatmeal milk = 10 |
| Melting | Dissolves in the mouth and the structure disappears, like ice cream melting. | Water = 0; Ice cream = 10 |
| Spreadability | Unctuous, like chocolate spread. | Milk = 0; Chocolate sauce = 10 |
| Thickness | Sample viscosity on the tongue. Sample is “flowing” if it flows immediately over the tongue. The sample is “sticky” if it stays on the tongue or flows slowly and is difficult to swallow. | Skim milk = 0; Cream = 3; Peanut butter = 10 |
| Smoothness | The perception of lubricity or graininess when the sample is weakly compressed between the tongue and the palate and the tongue rubs repeatedly on the palate. The sample is “smooth” if it feels smooth and has no rough particles. The sample is “rough” if rough or irregular particles are felt in the mouth. | Peanut butter = 0; Skim milk = 3; Jelly pudding = 10 |
| Mouth coating | The degree to which the coating is felt throughout the mouth; it can be felt on the teeth and upper jaw. | Whole milk = 5; Butter = 10 |
| Overall creaminess | Cream is a soft, full feeling, and scores are based on a comprehensive feeling. | Skim milk = 0; Marshmallow sauce = 3; Cream = 10 |
| EG10 | EG20 | EG40 | EG60 | EG85 | |
|---|---|---|---|---|---|
| Solid fat content | 10.61 ± 0.41 a | 23.98 ± 0.86 b | 42.57 ± 0.12 c | 62.27 ± 0.38 d | 85.87 ± 0.13 e |
| η150/s (Pa s) | 1.65 ± 0.04 a | 1.60 ± 0.05 a | 1.61 ± 0.04 a | 1.74 ± 0.04 a,b | 1.86 ± 0.07 b |
| η250/s (Pa s) | 0.16 ± 0.02 a | 0.17 ± 0.01 a | 0.18 ± 0.02 a | 0.17 ± 0.01 a | 0.17 ± 0.01 a |
| μ20 | 0.34 ± 0.07 b | 0.26 ± 0.01 b | 0.12 ± 0.01 a | 0.28 ± 0.02 b | 0.31 ± 0.04 b |
| Descriptor | EG10 | EG20 | EG40 | EG60 | EG85 |
|---|---|---|---|---|---|
| Graininess | 3.25 ± 0.54 a | 3.13 ± 0.57 a | 3.35 ± 0.58 a | 3.55 ± 0.50 a | 3.65 ± 0.53 a |
| Melting | 3.42 ± 0.64 bc | 3.55 ± 0.50 bc | 4.00 ± 0.67 c | 3.00 ± 0.71 b | 2.06 ± 0.55 a |
| Spreadability | 5.07 ± 0.65 a | 5.56 ± 0.48 a | 5.60 ± 0.74 a | 5.38 ± 0.77 a | 4.88 ± 0.94 a |
| Thickness | 5.43 ± 0.55 a | 5.75 ± 0.48 a | 5.95 ± 0.60 a | 5.55 ± 0.60 a | 5.40 ± 0.52 a |
| Smoothness | 5.65 ± 0.53 a | 7.30 ± 0.59 b | 8.15 ± 0.47 c | 6.80 ± 0.63 b | 5.75 ± 0.59 a |
| Mouth coating | 5.95 ± 0.50 a | 6.70 ± 0.54 b | 7.65 ± 0.58 c | 6.30 ± 0.59 ab | 6.07 ± 0.72 a |
| Overall creaminess | 6.40 ± 0.48 a | 7.25 ± 0.54 b | 8.98 ± 0.54 c | 6.93 ± 0.69 ab | 6.53 ± 0.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhao, Y.; Fan, D.; Shen, Q.; Liu, C.; Luo, J. Effect of Solid Fat Content in Fat Droplets on Creamy Mouthfeel of Acid Milk Gels. Foods 2022, 11, 2932. https://doi.org/10.3390/foods11192932
Zhou H, Zhao Y, Fan D, Shen Q, Liu C, Luo J. Effect of Solid Fat Content in Fat Droplets on Creamy Mouthfeel of Acid Milk Gels. Foods. 2022; 11(19):2932. https://doi.org/10.3390/foods11192932
Chicago/Turabian StyleZhou, Hui, Yan Zhao, Di Fan, Qingwu Shen, Chengguo Liu, and Jie Luo. 2022. "Effect of Solid Fat Content in Fat Droplets on Creamy Mouthfeel of Acid Milk Gels" Foods 11, no. 19: 2932. https://doi.org/10.3390/foods11192932