A Smartphone Colorimetric Sensor Based on Pt@Au Nanozyme for Visual and Quantitative Detection of Omethoate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of Pt@Au Nanozymes, Signal Probe and Capture Probe
2.3. Development of Colorimetric Sensor
2.4. Sample Preparation
2.5. ELISA and GC Analysis
3. Results and Discussion
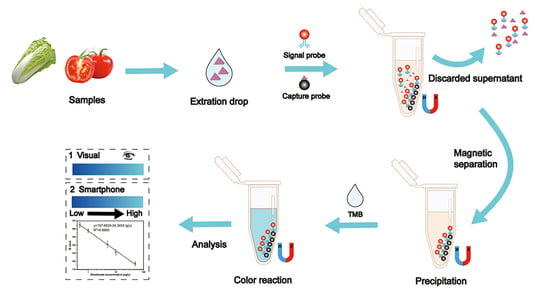
3.1. Principle of the Colorimetric Sensor
3.2. Characterization of Pt@Au Nanozyme
3.3. Development of Colorimetric Sensor
3.4. Evaluation of Specificity
3.5. Analytical Application in Food Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.P.; Lin, B.X.; Cao, Y.J.; Guo, M.L.; Yu, Y. Fluorescence Determination of Omethoate Based on a Dual Strategy for Improving Sensitivity. J. Agric. Food Chem. 2017, 65, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, D.; Ye, S. Detection of pesticide residue distribution on fruit surfaces using surface-enhanced Raman spectroscopy imaging. RSC Adv. 2018, 8, 4726–4730. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Guo, M.L.; Tan, J.A.; Geng, Y.Y.; Wu, J.Y.; Tang, Y.W.; Su, C.C.; Lin, C.C.; Liang, Y. Novel Fluorescence Sensor Based on All-Inorganic Perovskite Quantum Dots Coated with Molecularly Imprinted Polymers for Highly Selective and Sensitive Detection of Omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef] [PubMed]
- Herron, G.A.; Wilson, L.J. Can resistance management strategies recover insecticide susceptibility in pests?: A case study with cotton aphid Aphis gossypii (Aphididae: Hemiptera) in Australian cotton. Austral Entomol. 2017, 56, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Rao, Z.; Ji, H. NIR Hyperspectral Imaging Technology Combined with Multivariate Methods to Study the Residues of Different Concentrations of Omethoate on Wheat Grain Surface. Sensors 2019, 19, 3147. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.G.; Wang, Y.M.; Yan, S.L.; Ye, L.B. Optimization of omethoate degradation conditions and a kinetics model. Int. Biodeter. Biodegr. 2008, 62, 239–243. [Google Scholar]
- Gavahian, M.; Khaneghah, A.M. Cold plasma as a tool for the elimination of food contaminants: Recent advances and future trends. Crit. Rev. Food Sci. 2020, 60, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Wang, L.N.; Zhao, Y.; Wang, B.J. Identification and Dissipation of Omethoate and Its Main Metabolite DMP in Wheat Determined by UPLC-QTOF/MS. J. Agric. Food Chem. 2019, 67, 5891–5898. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Env. Sci. Tec. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Lazarevi Pašti, T.D.; Pašti, I.A.; Joki, B.; Babića, B.M.; Vasić, V.M. Heteroatom-doped mesoporous carbons as efficient adsorbents for removal of dimethoate and omethoate from water. RSC Adv. 2016, 6, 62128–62139. [Google Scholar] [CrossRef]
- Nair, R.V.; Chandran, P.R.; Mohamed, A.P.; Pillai, S. Sulphur-doped graphene quantum dot based fluorescent turn-on aptasensor for selective and ultrasensitive detection of omethoate. Anal. Chim. Acta 2021, 1181, 338893. [Google Scholar] [CrossRef] [PubMed]
- Bouagga, A.; Chaabane, H.; Toumi, K.; Hamdane, A.M.; Nasraoui, B.; Joly, L. Pesticide residues in Tunisian table grapes and associated risk for consumer’s health. Food Addit. Contam. B 2019, 12, 135–144. [Google Scholar] [CrossRef] [PubMed]
- GB/T 2763-2021; National Food Safety Standard Maximum Residue Limits of Pesticides in Foods (Print Version). China Agricultural Press: Beijing, China, 2021. Available online: http://down.foodmate.net/standard/sort/3/97819.html (accessed on 1 September 2022).
- Huo, Q.; Quan, Q.; Tao, F.; Wang, Y. Controlling the Red Spider Mite, Tetranychus urticae Koch with Ailanthus altissima Leaf Extract. Egypt. J. Biol. Pest Control 2016, 26, 671–674. [Google Scholar]
- Zheng, L.L.; Pi, F.W.; Wang, Y.F.; Xu, H.; Zhang, Y.Z.; Sun, X.L. Photocatalytic degradation of Acephate, Omethoate, and Methyl parathion by Fe3O4@SiO2@mTiO2 nanomicrospheres. J. Hazard. Mater. 2016, 315, 11–22. [Google Scholar] [CrossRef]
- Yu, R.; Wang, Y.; Cui, Z.W.; Xu, G.H.; Guan, Z.Y.; Yu, Y.; Liu, J.S. Human health risk assessment of organophosphorus pesticides in maize (Zea mays L.) from Yushu, Northeast China. Hum. Ecol. Risk Assess. 2018, 24, 642–652. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al Sallagi, M.S.; Dhanasekaran, D.K.; Odeh, W.A.M.B.; Al Ali, H.J.; Al Ali, A.A.S.A.; Radwan, H.; Obaid, R.S.; Holley, R. Pesticide residues in fresh vegetables imported into the United Arab Emirates. Food Control 2021, 133, 108663. [Google Scholar] [CrossRef]
- Pasar, R.; Pallavi, M.S.; Naik, R.H.; Devaraj, M.; Nandini, P.; Bheemanna, M.; Badariprasad, P.R.; Paramasivam, M. Simultaneous determination of dimethoate and its metabolite omethoate in curry leaf using LC-MS/MS and risk assessment. J. Sep. Sci. 2022, 45, 1831–1838. [Google Scholar] [CrossRef]
- Tian, F.Y.; Zhou, J.; Jiao, B.N.; He, Y. A nanozyme-based cascade colorimetric aptasensor for amplified detection of ochratoxin A. Nanoscale 2019, 11, 9547–9555. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Li, M.F.; Guo, C.Y.; Jia, Z.; Wan, G.C.; Wang, S.F.; Min, D.Y. Fe3O4Nanoparticles Loaded on Lignin Nanoparticles Applied as a Peroxidase Mimic for the Sensitively Colorimetric Detection of H2O2. Nanomaterials 2019, 9, 210. [Google Scholar] [CrossRef]
- Zhang, Q.T.; Yu, Y.Y.; Yun, X.J.; Luo, B.; Jiang, H.R.; Chen, C.Z.; Wang, S.F.; Min, D.Y. Multicolor Colorimetric Sensor for Detection of Omethoate Based on the Inhibition of the Enzyme-Induced Metallization of Gold Nanorods. ACS Appl. Nano Mater. 2020, 3, 5212–5219. [Google Scholar] [CrossRef]
- Zhang, X.D.; Chen, X.K.; Zhao, Y.L. Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy. Nano-Micro Lett. 2022, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.X.; Wang, X.Y.; Wang, Q.; Lou, Z.P.; Li, S.R.; Zhu, Y.Y.; Li, Q.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Nextgeneration artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Wei, H. Nanozymes in bionanotechnology: From sensing to therapeutics and beyond. Inorg. Chem. Front. 2016, 3, 41–60. [Google Scholar] [CrossRef]
- Zhong, H.T.; Xue, Y.T.; Zhang, P.Y.; Liu, B.; Zhang, X.; Chen, Z.B.; Li, K.; Zheng, L.R.; Zuo, X. Cascade reaction system integrating nanozymes for colorimetric discrimination of organophosphorus pesticides. Sens. Actuators B-Chem. 2022, 350, 130810. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Nanozymes: A Promising Horizon for Medical and Environmental Applications. J. Clust. Sci. 2022, 33, 1275–1297. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Imura, M.; Yamauchi, Y. All-Metal Mesoporous Nanocolloids: Solution-Phase Synthesis of Core-Shell Pd@Pt Nanoparticles with a Designed Concave Surface. Angew. Chem. Int. Ed. 2013, 52, 13611–13615. [Google Scholar] [CrossRef]
- Torres-Pacheco, L.J.; De Leon-Rodriguez, A.; Alvarez-Contreras, L.; Guerra-Balcazar, M.; Arjona, N. Sorbitol electro-oxidation reaction on sub <10 nm PtAu bimetallic Nanoparticles. Electrochim. Acta 2020, 353, 136593. [Google Scholar]
- Oujji, N.B.; Bakas, I.; Istamboulie, G.; Ait-Ichou, I.; Ait-Addi, E.; Rouillon, R.; Noguer, T. A Simple Colorimetric Enzymatic-Assay, based on immobilization of acetylcholinesterase by adsorption, for sensitive detection of organophosphorus insecticides in olive oil. Food Control 2014, 46, 75–80. [Google Scholar]
- Han, Z.; Chi, C.S.; Bai, B.; Liu, G.; Rao, Q.X.; Peng, S.J.; Liu, H.; Zhao, Z.H.; Zhang, D.B.; Wu, A.B. Chromogenic platform based on recombinant drosophila melanogaster acetylcholinesterase for visible unidirectional assay of organophosphate and carbamate insecticide residues. Anal. Chim. Acta 2012, 720, 126–133. [Google Scholar]
- Guo, X.S.; Zhang, X.Y.; Cai, Q.; Shen, T.; Zhu, S.M. Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food. Food Control 2013, 30, 15–23. [Google Scholar]
- Pang, S.; Labuza, T.P.; He, L. Development of a single aptamer-based surface enhanced raman scattering method for rapid detection of multiple pesticides. Analyst 2014, 139, 1895–1901. [Google Scholar]





| Samples | Added (μg/kg) | This Colorimetric Sensor | ELISA | GC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Detected (μg/kg) | Recovery (%) | CV (%) | Detected (μg/kg) | Recovery (%) | CV (%) | Detected (μg/kg) | Recovery (%) | CV (%) | ||
| Chinese cabbage | 0 | ND | - | - | ND | - | - | ND | - | - |
| 20 | 17.67 | 88.33 | 10.92 | 18.40 | 92.00 | 4.10 | 19.03 | 95.17 | 2.12 | |
| 200 | 178.83 | 89.42 | 9.61 | 177.97 | 88.98 | 6.02 | 189.50 | 94.75 | 3.71 | |
| 1000 | 902.00 | 90.20 | 5.16 | 895.60 | 89.56 | 6.19 | 905.20 | 90.52 | 4.31 | |
| Tomato | 0 | ND | - | - | ND | - | - | ND | - | - |
| 20 | 17.70 | 88.50 | 11.47 | 17.53 | 87.67 | 5.71 | 17.51 | 87.57 | 4.33 | |
| 200 | 172.30 | 86.15 | 8.13 | 168.83 | 84.42 | 5.78 | 177.83 | 88.92 | 3.37 | |
| 1000 | 803.47 | 80.35 | 6.02 | 854.80 | 85.48 | 6.15 | 897.79 | 89.78 | 3.23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhou, R.; Zhang, H.; Cai, D.; Lin, X.; Lang, Y.; Qiu, Y.; Shentu, X.; Ye, Z.; Yu, X. A Smartphone Colorimetric Sensor Based on Pt@Au Nanozyme for Visual and Quantitative Detection of Omethoate. Foods 2022, 11, 2900. https://doi.org/10.3390/foods11182900
Zhang B, Zhou R, Zhang H, Cai D, Lin X, Lang Y, Qiu Y, Shentu X, Ye Z, Yu X. A Smartphone Colorimetric Sensor Based on Pt@Au Nanozyme for Visual and Quantitative Detection of Omethoate. Foods. 2022; 11(18):2900. https://doi.org/10.3390/foods11182900
Chicago/Turabian StyleZhang, Biao, Ruofan Zhou, Huiqi Zhang, Danfeng Cai, Xiaodong Lin, Yihan Lang, Yulou Qiu, Xuping Shentu, Zihong Ye, and Xiaoping Yu. 2022. "A Smartphone Colorimetric Sensor Based on Pt@Au Nanozyme for Visual and Quantitative Detection of Omethoate" Foods 11, no. 18: 2900. https://doi.org/10.3390/foods11182900