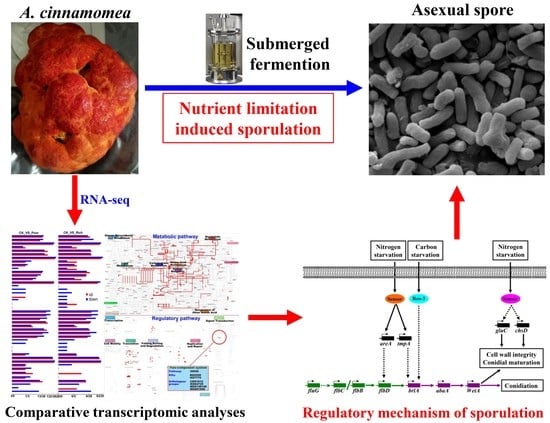
Comparative Transcriptomic Analyses Reveal the Regulatory Mechanism of Nutrient Limitation-Induced Sporulation of Antrodia cinnamomea in Submerged Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Medium
- The fermentation medium comprised the following: 20.0 g/L glucose, 100 g/L yeast extract powder, 3.0 g/L KH2PO4, 1.5 g/L MgSO4, and initial pH of 3.9 [15];
- The basal medium comprised the following: 15.0 g/L glucose, 5.0 g/L yeast extract powder, 1.44 g/L KH2PO4, 0.38 g/L MgSO4, and initial pH of 3.9.
2.3. Seed Preparation and Submerged Fermentation of A. cinnamomea
2.4. Detection Methods
- Detection of fermentation broth components: the fermentation broth cultured for 5 days was filtered with four layers of gauze to collect the filtrate. The glucose content was detected by a biosensor analyzer (SBA-40C, Shandong academy of sciences, China), the nitrogen content was detected by an element analyzer (VARIOEL III, Elementar Analysensyetem GmbH, Langenselbold, Germany), and the SO42− and PO43− content was detected by an atomic absorption spectrophotometer (DW-AA2081, Spectro, Kleve, Germany);
- Detection of contents of carbon and nitrogen in yeast extract powder: the element analyzer (VARIOEL III, Elementar, Langenselbold, Germany) was used for detection;
- Detection of the pH of fermentation broth: the pH meter (MettLer-ToLedo, Columbus, OH, USA) was used for detection [8];
- Detection of biomass: the fermentation broth was filtered with four layers of gauze. After the obtained mycelium pellets were washed with deionized water three times, they were dried at 75 °C to constant weight, weighted, and calculated for their biomass [8];
- Detection of sporulation: the spores were counted using a hemocytometer under an optical microscope, and the sporulation was then calculated [8].
2.5. Effects of Different Nutritional Conditions on Sporulation of A. cinnamomea
2.5.1. Effects of Different Contents of Yeast Extract Powder on Sporulation of A. cinnamomea
2.5.2. Effects of Different Contents of Glucose on Sporulation of A. cinnamomea
2.6. Sample Preparation and RNA Extraction of A. cinnamomea Mycelia
2.6.1. Sample Preparation of Mycelia
2.6.2. Total RNA Extraction
2.7. RNA-Seq and Bioinformatic Analysis
2.8. RT-qPCR Analysis
2.9. Statistical Analysis of Data
3. Results and Discussion
3.1. Components of Fermentation Broth Cultured for 5 Days
3.2. Effects of Different Nutritional Conditions on the Asexual Sporulation of A. cinnamomea
3.3. RNA-Seq and Statistical Analysis
3.3.1. Statistical Analysis of Sample Repeatability and Differentially Expressed Genes
3.3.2. GO Functional Analysis of Differentially Expressed Genes
3.3.3. iPath Analysis of Differentially Expressed Genes
3.4. Bioinformatic Analysis
3.5. RT-qPCR Analysis
3.6. Model Diagram of A. cinnamomea Asexual Sporulation Signal Pathway Induced by Nutrition Limitation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chen, C.L.; Li, W.C.; Chuang, Y.C.; Liu, H.C.; Huang, C.H.; Lo, K.Y.; Chen, C.Y.; Chang, F.M.; Chang, G.A.; Lin, Y.L.; et al. Sexual crossing, chromosome-level genome sequences, and comparative genomic analyses for the medicinal mushroom Taiwanofungus camphoratus (Syn. Antrodia Cinnamomea, Antrodia Camphorata). Microbiol. Spectr. 2022, 10, e0203221. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Lin, J.; Lim, S.; Cao, Y.; Chen, S.; Xu, P.; Xu, C.; Zheng, H.; Fu, K.C.; et al. Structure and anti-inflammatory activity relationship of ergostanes and lanostanes in Antrodia cinnamomea. Foods 2022, 11, 1831. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Geng, Y.; Xu, H.X.; Ren, Y.; Liu, D.Y.; Mao, Y. Antrodia camphorata-derived antrodin C inhibits liver fibrosis by blocking TGF-Beta and PDGF signaling pathways. Front. Mol. Biosci. 2022, 9, 835508. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.K.; Wu, Y.C.; Yeh, T.S.; Hsieh, C.R.; Tsai, Y.H.; Wei, C.K.; Li, C.Y.; Lu, Y.C.; Chang, F.R. Regulatory effect of intracellular polysaccharides from Antrodia cinnamomea on the intestinal microbiota of mice with antibiotic-associated diarrhea. Qual. Assur. Saf. Crop. 2022, 14, 124–134. [Google Scholar] [CrossRef]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of freeze-dried mycelia of Antrodia camphorata as a novel food pursuant to regulation (EU) 2015/2283. EFSA J. 2022, 20, e07380. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Geng, Y.; Gong, J.S.; Zhang, X.J.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Efficient production of bioactive metabolites from Antrodia camphorata ATCC 200183 by asexual reproduction-based repeated batch fermentation. Bioresour. Technol. 2015, 194, 334–343. [Google Scholar] [CrossRef]
- Hu, Y.D.; Lu, R.Q.; Liao, X.R.; Zhang, B.B.; Xu, G.R. Stimulating the biosynthesis of antroquinonol by addition of effectors and soybean oil in submerged fermentation of Antrodia camphorata. Appl. Biochem. Biotechnol. 2016, 63, 398–406. [Google Scholar] [CrossRef]
- Xia, Y.J.; Zhou, X.; Liang, L.; Liu, X.; Li, H.; Xiong, Z.; Wang, G.; Song, X.; Ai, L. Genetic evidence for the requirements of antroquinonol biosynthesis by Antrodia camphorata during liquid-state fermentation. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab086. [Google Scholar] [CrossRef]
- Meng, L.; Luo, B.; Yang, Y.; Faruque, M.O.; Zhang, J.; Li, X.; Hu, X. Addition of vegetable oil to improve triterpenoids production in liquid fermentation of medicinal fungus Antrodia cinnamomea. J. Fungi 2021, 7, 926. [Google Scholar] [CrossRef]
- Lu, Z.M.; He, Z.; Li, H.X.; Gong, J.S.; Geng, Y.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Modified arthroconidial inoculation method for the efficient fermentation of Antrodia camphorata ATCC 200183. Biochem. Eng. J. 2014, 87, 41–49. [Google Scholar] [CrossRef]
- Liu, H.; Xing, H.; Jin, Y.; Liu, J.; Tzeng, Y.M.; Deng, L.; Wang, F. Application of multiple strategies to improve the production of the potential cancer drug 4-acetylantroquinonol B (4-aaqb) by the rare fungus Antrodia cinnamomea. Appl. Biochem. Biotechnol. 2022, 194, 2720–2730. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, Y.; Zhang, Y.; Yi, Z.; Meng, P.; Wang, G.; Ai, L. Enhancement of antroquinonol and antrodin C productions via in situ extractive fermentation of Antrodia camphorata S-29. Appl. Microbiol. Biotechnol. 2019, 103, 8351–8361. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.M.; Zhu, Q.; Gong, J.S.; Geng, Y.; Shi, J.S.; Xu, Z.H.; Ma, Y.H. Comparative transcriptomic and proteomic analyses reveal a flug-mediated signaling pathway relating to asexual sporulation of Antrodia camphorata. Proteomics 2017, 17, 1700256. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆t Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; He, Z.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Antrodia camphorata ATCC 200183 sporulates asexually in submerged culture. Appl. Microbiol. Biotechnol. 2013, 97, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Yu, J.H. Developmental regulators in Aspergillus fumigatus. J. Microbiol. 2016, 54, 223–231. [Google Scholar] [CrossRef]
- McCormick, J.R.; Flardh, K. Signals and regulators that govern Streptomyces development. FEMS Microbiol. Rev. 2012, 36, 206–231. [Google Scholar] [CrossRef]
- Resch, U.; Tsatsaronis, J.A.; Le Rhun, A.; Stubiger, G.; Rohde, M.; Kasvandik, S.; Holzmeister, S.; Tinnefeld, P.; Wai, S.N.; Charpentier, E. A two-component regulatory system impacts extracellular membrane-derived vesicle production in Group A Streptococcus. Am. Soc. Microbiol. 2016, 7, E00207-16. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Ma, W.B.; Li, Y.; Wang, H.; Que, Y.W.; Ma, Z.H.; Talbot, N.J.; Wang, Z.Y. A sterol 14α-demethylase is required for conidiation, virulence and for mediating sensitivity to sterol demethylation inhibitors by the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 2011, 48, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Daryaei, A.; Jones, E.E.; Glare, T.R.; Falloon, R.E. Nutrient amendments affect Trichoderma atroviride conidium production, germination and bioactivity. Biol. Control 2016, 93, 8–14. [Google Scholar] [CrossRef]
- Roncal, T.; Ugalde, U. Conidiation induction in Penicillium. Res. Microbiol. 2003, 154, 539–546. [Google Scholar] [CrossRef]
- Ozcan, S.; Dover, J.; Rosenwald, A.G.; Wolfl, S.; Johnston, M. Two glucose transporters in Saccharomyces cerevisiae are glucose sensors that generate a signal for induction of gene expression. Proc. Natl. Acad. Sci. USA 1996, 93, 12428–12432. [Google Scholar] [CrossRef]
- Madi, L.; McBride, S.A.; Bailey, L.A.; Ebbole, D.J. rco-3, a gene involved in glucose transport and conidiation in Neurospora crassa. Genetics 1997, 146, 499–508. [Google Scholar] [CrossRef]
- van Munster, J.M.; Dobruchowska, J.M.; Veloo, R.; Dijkhuizen, L.; van der Maarel, M.J. Characterization of the starvation-induced chitinase CfcA and α-1,3-glucanase AgnB of Aspergillus niger. Appl. Microbiol. Biotechnol. 2015, 99, 2209–2223. [Google Scholar] [CrossRef]
- Katz, M.E.; Buckland, R.; Hunter, C.C.; Todd, R.B. Distinct roles for the p53-like transcription factor XprG and autophagy genes in the response to starvation. Fungal Genet. Biol. 2015, 83, 10–18. [Google Scholar] [CrossRef]
- Lu, H.Y.; Huang, Y.L.; Wu, P.C.; Wei, X.Y.; Yago, J.I.; Chung, K.R. A zinc finger suppressor involved in stress resistance, cell wall integrity, conidiogenesis, and autophagy in the necrotrophic fungal pathogen Alternaria alternata. Microbiol. Res. 2022, 263, 127106. [Google Scholar] [CrossRef]
- Lin, L.L.; Cao, J.Y.; Du, A.Q.; An, Q.L.; Chen, X.M.; Yuan, S.S.; Batool, W.; Shabbir, A.; Zhang, D.M.; Wang, Z.H.; et al. eIF3k domain-containing protein regulates conidiogenesis, appressorium turgor, virulence, stress tolerance, and physiological and pathogenic development of Magnaporthe oryzae. Front. Plant Sci. 2021, 12, 748120. [Google Scholar] [CrossRef]
- Szilágyi, M.; Kwon, N.J.; Bakti, F.; M-Hamvas, M.; Jámbrik, K.; Park, H.; Pócsi, I.; Yu, J.H.; Emri, T. Extracellular proteinase formation in carbon starving Aspergillus nidulans cultures—Physiological function and regulation. J. Basic Microbiol. 2011, 51, 625–634. [Google Scholar] [CrossRef]
- Hou, J.; Wang, J.J.; Lin, H.Y.; Feng, M.G.; Ying, S.H. Roles of autophagy-related genes in conidiogenesis and blastospore formation, virulence, and stress response of Beauveria bassiana. Fungal Biol. 2020, 124, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Brunner, K.; Montero, M.; Mach, R.L.; Peterbauer, C.K.; Kubicek, C.P. Expression of the ech42 (endochitinase) gene of Trichoderma atroviride under carbon starvation is antagonized via a BrlA-like cis-acting element. FEMS Microbiol. Lett. 2003, 218, 259–264. [Google Scholar] [CrossRef] [PubMed]
- van Munster, J.M.; Nitsche, B.M.; Akeroyd, M.; Dijkhuizen, L.; van der Maarel, M.J.; Ram, A.F. Systems approaches to predict the functions of glycoside hydrolases during the life cycle of Aspergillus niger using developmental mutants ∆brlA and ∆flbA. PLoS ONE 2015, 10, E0116269. [Google Scholar] [CrossRef] [PubMed]
- Soid-Raggi, G.; Sanchez, O.; Aguirre, J. TmpA, a member of a novel family of putative membrane flavoproteins, regulates asexual development in Aspergillus nidulans. Mol. Microbiol. 2006, 59, 854–869. [Google Scholar] [CrossRef]
- Todd, R.B.; Fraser, J.A.; Wong, K.H.; Davis, M.A.; Hynes, M.J. Nuclear accumulation of the GATA factor AreA in response to complete nitrogen starvation by regulation of nuclear export. Eukaryot Cell 2005, 4, 1646–1653. [Google Scholar] [CrossRef]
- Portnoy, T.; Margeot, A.; Linke, R.; Atanasova, L.; Fekete, E.; Sandor, E.; Hartl, L.; Karaffa, L.; Druzhinina, I.S.; Seiboth, B.; et al. The CRE1 carbon catabolite repressor of the fungus Trichoderma reesei: A master regulator of carbon assimilation. BMC Genom. 2011, 12, 269. [Google Scholar] [CrossRef]
- Skromne, I.; Sánchez, O.; Aguirre, J. Starvation stress modulates the expression of the Aspergillus nidulans brlA regulatory gene. Microbiology 1995, 141, 21–28. [Google Scholar] [CrossRef]
- Arratia-Quijada, J.; Sanchez, O.; Scazzocchio, C.; Aguirre, J. FlbD, a Myb transcription factor of Aspergillus nidulans, is uniquely involved in both asexual and sexual differentiation. Eukaryot Cell 2012, 11, 1132–1142. [Google Scholar] [CrossRef]
- Park, H.S.; Yu, J.H. Genetic control of asexual sporulation in filamentous fungi. Curr. Opin. Microbiol. 2012, 15, 669–677. [Google Scholar] [CrossRef]
- Oiartzabal-Arano, E.; Perez-de-Nanclares-Arregi, E.; Espeso, E.A.; Etxebeste, O. Apical control of conidiation in Aspergillus nidulans. Curr. Genet. 2016, 62, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Jin, K.; Xia, Y. Transcriptional analysis of the conidiation pattern shift of the entomopathogenic fungus Metarhizium acridum in response to different nutrients. BMC Genom. 2016, 17, 586. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Wang, J.J.; Feng, M.G.; Ying, S.H. Autophagy-related gene ATG7 participates in the asexual development, stress response and virulence of filamentous insect pathogenic fungus Beauveria bassiana. Curr. Genet. 2019, 65, 1015–1024. [Google Scholar] [CrossRef] [PubMed]





| Gene Name | Upstream Primer (5′→3′) | Downstream Primer (5′→3′) | Product (bp) |
|---|---|---|---|
| rco3 | GCCGCCGACTCCCTCTG | AATGAACAAGCAAGCACTGACG | 140 |
| tmpA | GGCAGAAATTCCAAGAGCATAGTC | CACCTCAGGCACAACCATCC | 178 |
| areA | GTAGAGTGAGGCAAGGCAGATG | TGTCCAATTCAGTCCGCATACC | 139 |
| atg5 | GGCGAGGAGGTATAATATGAGTGG | GGTGACGAAGGAGCGAAAGC | 152 |
| ech42 | TCGCTGTATTTCTGTCGCTCAC | GCACCAACACCGCTCTACC | 132 |
| gluC | CAGGCGAGATGGTAAGGATACG | AGCGGGAAATGATGGTGAGC | 141 |
| eng1 | GAGGAGGATTGGATGCGGATG | AACGAGGTCTCTAACAGTGATGC | 161 |
| proteinase | AGAAAGGGTGGTCAAGGTTATGC | CAGAAGAGGAGGTGCGAGATTAC | 175 |
| cre1 | TCTCTTCGCTTGGTTCACATCC | CGCTTGTATATGGCTCCTGGTC | 180 |
| chsD | ATATGGTATGTGGAGTGGTTGGC | GGCGGTGGCGATGATTGG | 175 |
| CYP51A | GTGTTGGAAATGTTGACGAGGAC | GCGGAGTGGAAGATGACAAGG | 178 |
| 18S rRNA | GCTGGTCGCTGGCTTCTTAG | CGCTGGCTCTGTCAGTGTAG | 123 |
| Component | Content (g/L) | |
|---|---|---|
| 0 Day | 5 Days | |
| glucose | 20.1 ± 0.2 | 15.1 ± 1.4 |
| nitrogen | 1.2 ± 0.1 | 0.6 ± 0.0 |
| SO42− | 1.2 ± 0.0 | 0.3 ± 0.0 |
| PO43− | 2.1 ± 0.1 | 1.0 ± 0.1 |
| pH | 4.5 ± 0.0 | 3.9 ± 0.1 |
| Content (g/L) | Biomass (g/L) | Sporulation (×105 Spores/mL) | Sporulation Capability (×108 Spores/g) | Residual Sugar (g/L) |
|---|---|---|---|---|
| Nitrogen (yeast extract powder) contents (g/L) | ||||
| 0 | 3.6 ± 0.3 b | 230.0 ± 20.3 f | 63.5 ± 2.7 f | 4.9 ± 0.1 a |
| 0.5 | 4.0 ± 0.3 bc | 63.5 ± 4.5 e | 15.8 ± 1.0 e | 4.7 ± 0.1 a |
| 1.0 | 4.0 ± 0.3 bc | 35.5 ± 3.3 d | 8.9 ± 0.6 d | 4.7 ± 0.1 a |
| 5.0 | 4.6 ± 0.3 cd | 8.3 ± 0.3 c | 1.8 ± 0.1 c | 4.6 ± 0.1 a |
| 10 | 4.9 ± 0.4 d | 4.0 ± 0.5 b | 0.8 ± 0.0 b | 4.6 ± 0.2 a |
| Control | ||||
| CK1 | 4.8 ± 0.4 d | 2.8 ± 0.3 b | 0.6 ± 0.0 b | 4.5 ± 0.2 a |
| CK2 | 4.7 ± 0.4 d | 2.3 ± 0.3 b | 0.5 ± 0.0 b | 4.5 ± 0.1 a |
| 5 days | 1.5 ± 0.1 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 13.5 ± 1.2 b |
| Carbon (glucose) contents (g/L) | ||||
| 0 | 1.3 ± 0.1 a | 165.0 ± 12.5 d | 132.0 ± 8.7 e | 0.1 ± 0.0 a |
| 2.0 | 1.9 ± 0.2 b | 262.5 ± 25.2 e | 135.3 ± 9.1 e | 0.1 ± 0.0 a |
| 5.0 | 2.8 ± 0.3 c | 487.5 ± 30.5 f | 176.6 ± 9.7 f | 0.1 ± 0.0 a |
| 10 | 3.0 ± 0.1 c | 256.3 ± 13.7 e | 84.6 ± 4.2 d | 0.2 ± 0.0 a |
| 20 | 3.9 ± 0.3 d | 114.3 ± 8.0 c | 29.1 ± 2.0 c | 8.9 ± 0.8 c |
| Control | ||||
| CK1 | 4.4 ± 0.2 e | 4.3 ± 0.3 b | 0.9 ± 0.0 b | 4.6 ± 0.1 b |
| CK2 | 4.3 ± 0.3 e | 4.5 ± 0.2 b | 1.0 ± 0.0 b | 4.5 ± 0.2 b |
| 5 days | 1.8 ± 0.1 b | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 15.1 ± 1.4 d |
| Unigene ID | Genome ID | Gene Name | Accession Number | E Value | Homology (%) | Coverage (%) |
|---|---|---|---|---|---|---|
| c6157_g2 | ACg005466 | flbC | OAS999571 | 3.40 × 10−10 | 47.458 | 8 |
| c9703_g1 | ACg001829 | flbD | EEQ33126.1 | 7.23 × 10−25 | 43.802 | 31 |
| c5765_g1 | ACg000119 | brlA | AAM95989.1 | 5.33 × 10−11 | 53.846 | 12 |
| c6072_g2 | ACg005882 | vosA | XP_0095505191 | 5.54 × 10−59 | 39.61 | 88 |
| c2971_g1 | ACg005363 | pkaA | EFL410741 | 6.84 × 10−29 | 29.032 | 65 |
| c6203_g1 | ACg005139 | rco-3 | OBZ74137.1 | 5.25 × 10−106 | 36.154 | 94 |
| c5652_g1 | ACg002449 | tmpA | AAP13095.2 | 3.48 × 10−99 | 38.318 | 88 |
| c6469_g1 | ACg003505 | areA | CCO35477.1 | 6.08 × 10−32 | 37.908 | 25 |
| c7055_g1 | ACg003525 | atg5 | KYQ43884.1 | 2.56 × 10−180 | 71.023 | 97 |
| c4436_g1 | ACg008078 | ech42 | ABS82797.1 | 6.74 × 10−28 | 26.879 | 70 |
| c6333_g2 | ACg007557 | gluC | EHK46811.1 | 7.11 × 10−142 | 47.992 | 32 |
| c5534_g2 | ACg005514 | eng1 | AHI42991.1 | 6.44 × 10−149 | 62.776 | 99 |
| c6649_g1 | ACg000307 | proteasome | CEL57564.1 | 5.21 × 10−157 | 82.609 | 99 |
| c5803_g1 | ACg008021 | cre1 | AAT34979.1 | 4.24 × 10−14 | 51.563 | 14 |
| c4252_g1 | ACg006463 | chsD | CDM33077.1 | 2.03 × 10−14 | 21.918 | 26 |
| c6977_g1 | - | CYP51A | AIF79427.1 | 5.06 × 10−10 | 22.68 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ji, D.; Luo, Z.; Ren, Y.; Lu, Z.; Yang, Z.; Xu, Z. Comparative Transcriptomic Analyses Reveal the Regulatory Mechanism of Nutrient Limitation-Induced Sporulation of Antrodia cinnamomea in Submerged Fermentation. Foods 2022, 11, 2715. https://doi.org/10.3390/foods11172715
Li H, Ji D, Luo Z, Ren Y, Lu Z, Yang Z, Xu Z. Comparative Transcriptomic Analyses Reveal the Regulatory Mechanism of Nutrient Limitation-Induced Sporulation of Antrodia cinnamomea in Submerged Fermentation. Foods. 2022; 11(17):2715. https://doi.org/10.3390/foods11172715
Chicago/Turabian StyleLi, Huaxiang, Dan Ji, Zhishan Luo, Yilin Ren, Zhenming Lu, Zhenquan Yang, and Zhenghong Xu. 2022. "Comparative Transcriptomic Analyses Reveal the Regulatory Mechanism of Nutrient Limitation-Induced Sporulation of Antrodia cinnamomea in Submerged Fermentation" Foods 11, no. 17: 2715. https://doi.org/10.3390/foods11172715