Controlling the Interaction between Starchy Polyelectrolyte Layers for Adjusting Protein Release from Nanocapsules in a Simulated Gastrointestinal Tract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Multi-Layer Nanocapsule-Encapsulated Protein
2.3. Structural Properties of the Multi-Layer Nanocapsule
2.4. In Vitro Release
2.5. Characterization of the Quintet-Layer Nanocapsule in Simulated GITs
2.6. Statistical Analysis
3. Results
3.1. Characterization of Nanocapsules with Various Layers
3.2. In Vitro Release Profile
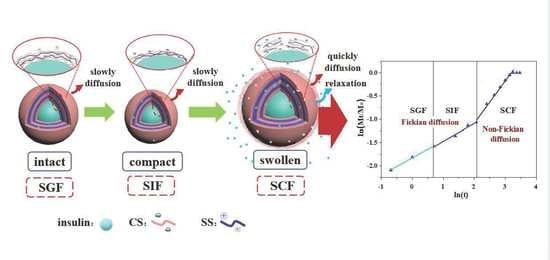
3.3. Release Kinetics of Nanocapsules in Simulated GIT
3.4. Structural Properties of Starch Polyelectrolytes and Quintet-Layer Nanocapsule in Simulated GIT
3.4.1. Interaction between Starch Polyelectrolytes in Simulated GIT
3.4.2. Structural Properties of Quintet-Layer Nanocapsule in Simulated GIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Daliri, E.B.-M.; Elahi, F.; Yeon, S.-J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.-H. The role of bioactive peptides in diabetes and obesity. Foods 2021, 10, 2220. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Zhang, Z.; Zhang, A.P.; Chen, L.; He, C.L.; Zhuang, X.L.; Chen, X.S. Novel thermo- and pH-responsive hydroxypropyl cellulose- and poly (L-glutamic acid)-based microgels for oral insulin controlled release. Carbohydr. Polym. 2012, 89, 1207–1214. [Google Scholar] [CrossRef]
- Situ, W.; Li, X.; Liu, J.; Chen, L. Preparation and characterization of glycoprotein-resistant starch complex as a coating material for oral bioadhesive microparticles for colon-targeted polypeptide delivery. J. Agric. Food Chem. 2015, 63, 4138–4147. [Google Scholar] [CrossRef]
- Yu, X.; Wen, T.; Cao, P.; Shan, L.; Li, L. Alginate-chitosan coated layered double hydroxide nanocomposites for enhanced oral vaccine delivery. J. Colloid Interface Sci. 2019, 556, 258–265. [Google Scholar] [CrossRef]
- Zhang, Y.; Chi, C.; Huang, X.; Zou, Q.; Li, X.; Chen, L. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract. Carbohydr. Polym. 2017, 171, 242–251. [Google Scholar] [CrossRef]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C. Preparation, Characteristics, and Advantages of Plant Protein-Based Bioactive Molecule Delivery Systems. Foods 2022, 11, 1562. [Google Scholar] [CrossRef]
- Velk, N.; Uhlig, K.; Vikulina, A.; Duschl, C.; Volodkin, D. Mobility of lysozyme in poly (l-lysine)/hyaluronic acid multilayer films. Colloids Surf. B Biointerfaces 2016, 147, 343–350. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Beyssac, E.; Garrait, G.; Hsein, H.; Cury, B.S.F. Retrograded starch/pectin coated gellan gum-microparticles for oral administration of insulin: A technological platform for protection against enzymatic degradation and improvement of intestinal permeability. Eur. J. Pharm. Biopharm. 2018, 123, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, J.; Raichur, A.M. Designing carboxymethyl cellulose based layer-by-layer capsules as a carrier for protein delivery. Colloids Surf. B Biointerfaces 2013, 101, 487–492. [Google Scholar] [CrossRef]
- Deshmukh, P.K.; Ramani, K.P.; Singh, S.S.; Tekade, A.R.; Bari, S.B. Stimuli-sensitive layer-by-layer (LbL) self-assembly systems: Targeting and biosensory applications. J. Control. Release 2013, 166, 294–306. [Google Scholar] [CrossRef]
- De Temmerman, M.L.; Demeester, J.; De Vos, F.; De Smedt, S.C. Encapsulation performance of layer-by-layer microcapsules for proteins. Biomacromolecules 2011, 12, 1283–1289. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Film. 1992, 210, 831–835. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Nagaraja, V.; Raichur, A.M. Reversible polyelectrolyte capsules as carriers for protein delivery. Colloids Surf. B Biointerfaces 2010, 78, 266–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, S.; Chi, C.; He, Y.; Li, X.; Chen, L.; Miao, S. Tailoring assembly behavior of starches to control insulin release from layer-by-layer assembled colloidal particles. Int. J. Biol. Macromol. 2020, 160, 531–537. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Zhang, B.; Xie, F. Starch-based materials encapsulating food ingredients: Recent advances in fabrication methods and applications. Carbohydr. Polym. 2021, 270, 118358. [Google Scholar] [CrossRef]
- Situ, W.; Chen, L.; Wang, X.; Li, X. Resistant starch film-coated microparticles for an oral colon-specific polypeptide delivery system and its release behaviors. J. Agric. Food Chem. 2014, 62, 3599–3609. [Google Scholar] [CrossRef]
- Zhang, Z.; Shan, H.; Chen, L.; He, C.; Zhuang, X.; Chen, X. Synthesis of pH-responsive starch nanoparticles grafted poly (l-glutamic acid) for insulin controlled release. Eur. Polym. J. 2013, 49, 2082–2091. [Google Scholar] [CrossRef]
- Li, Y.; Kleijn, M.; Slaghek, T.; Timmermans, J.; Stuart, M.C.; Norde, W. Lysozyme uptake and release by oxidized starch polymer microgels. J. Control. Release 2010, 148, e45–e46. [Google Scholar] [CrossRef]
- Humblet-Hua, K.; Scheltens, G.; Van Der Linden, E.; Sagis, L. Encapsulation systems based on ovalbumin fibrils and high methoxyl pectin. Food Hydrocoll. 2011, 25, 569–576. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the effect of freeze-drying on microstructures of starch hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Chen, L.; Li, L. Spermine modified starch-based carrier for gene delivery: Structure-transfection activity relationships. Carbohydr. Polym. 2017, 173, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Assaad, E.; Wang, Y.J.; Zhu, X.X.; Mateescu, M.A. Polyelectrolyte complex of carboxymethyl starch and chitosan as drug carrier for oral administration. Carbohydr. Polym. 2011, 84, 1399–1407. [Google Scholar] [CrossRef]
- Rafi, A.A.; Mahkam, M. Preparation of magnetic pH-sensitive microcapsules with an alginate base as colon specific drug delivery systems through an entirely green route. RSC Adv. 2015, 5, 4628–4638. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, H.; Huang, Z.; Zhu, J.; Wan, F.; Peng, T.; Pan, X.; Huang, Y.; Wu, C. Taste-masking and colloidal-stable cubosomes loaded with Cefpodoxime proxetil for pediatric oral delivery. Int. J. Pharm. 2020, 575, 118875. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, P.L.; Nima, J. Synthesis and characterization of novel drug delivery system using modified chitosan based hydrogel grafted with cyclodextrin. Chem. Eng. J. 2016, 284, 1259–1269. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Schaefer, D.W. Polymers, fractals, and ceramic materials. Science 1989, 243, 1023–1027. [Google Scholar] [CrossRef]
- Tan, J.P.; Wang, Q.; Tam, K.C. Control of burst release from nanogels via layer by layer assembly. J. Control. Release 2008, 128, 248–254. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Q.; Zhang, C.; Wu, H.; Liu, G.; Chen, R.; Guo, S. Controlled directional drug release from poly (ε-caprolactone)/polyethylene oxide/metoprolol tartrate composites with multi-layered structures. Compos. Part A Appl. Sci. Manuf. 2022, 157, 106939. [Google Scholar] [CrossRef]






| Nanocapsule | GIT | Kinetic Models | |||
|---|---|---|---|---|---|
| Zero-Order Release Model | Peppas Release Model | ||||
| K0 Value | R2 | n Value | R2 | ||
| Single-layer | SGF | 34.267 | 0.965 | 0.606 | 0.966 |
| SIF | – | – | – | – | |
| SCF | – | – | – | – | |
| Triplet-layer | SGF | 4.007 | 0.975 | 0.403 | 0.994 |
| SIF | 2.935 | 0.996 | 0.435 | 0.962 | |
| SCF | 3.405 | 0.993 | 0.732 | 0.971 | |
| Quintet-layer | SGF | 3.232 | 0.999 | 0.369 | 0.994 |
| SIF | 2.583 | 0.973 | 0.382 | 0.978 | |
| SCF | 3.692 | 0.981 | 0.787 | 0.994 | |
| pH 6.8 | pH 7.2 | |
|---|---|---|
| Ka (M-1) | 6.927 × 106 | 1.447 × 105 |
| ΔH (kJ/mol) | −23.8 | −15.2 |
| ΔS (J/mol·K) | 57.09 | 57.09 |
| TΔS (kJ/mol) | 14.16 | 14.13 |
| ΔG (kJ/mol) | −9.64 | −1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; He, Y.; Li, X. Controlling the Interaction between Starchy Polyelectrolyte Layers for Adjusting Protein Release from Nanocapsules in a Simulated Gastrointestinal Tract. Foods 2022, 11, 2681. https://doi.org/10.3390/foods11172681
Li Y, He Y, Li X. Controlling the Interaction between Starchy Polyelectrolyte Layers for Adjusting Protein Release from Nanocapsules in a Simulated Gastrointestinal Tract. Foods. 2022; 11(17):2681. https://doi.org/10.3390/foods11172681
Chicago/Turabian StyleLi, Yingying, Ying He, and Xiaoxi Li. 2022. "Controlling the Interaction between Starchy Polyelectrolyte Layers for Adjusting Protein Release from Nanocapsules in a Simulated Gastrointestinal Tract" Foods 11, no. 17: 2681. https://doi.org/10.3390/foods11172681