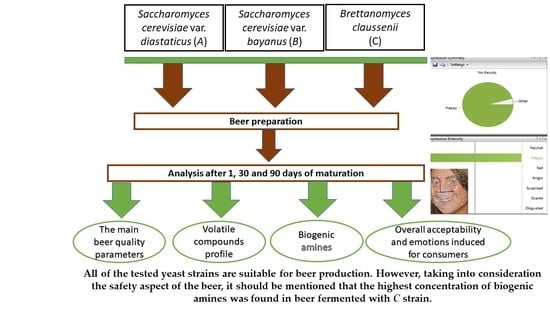
Effect of Different Yeast Strains on Biogenic Amines, Volatile Compounds and Sensory Profile of Beer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used for Beer Preparation
2.2. Beer Preparation
2.3. Evaluation of Beer Quality Parameters
2.4. Statistical Analysis
3. Results and Discussion
3.1. Beer Samples’ Quality Parameters
3.2. Overall Acceptability and Emotions Induced for Consumers by Beer Samples
3.3. Beer Volatile Compounds Profile
3.4. Biogenic Amines Concentration in Beer Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Beer Guide. Volume of World Beer Production. Available online: https://web.archive.org/web/20061028165040/http://www.europeanbeerguide.net/eustats.htm (accessed on 17 June 2022).
- Köster, E.P. Diversity in the determinants of food choice: A psychological perspective. Food Qual. Prefer. 2009, 20, 70–82. [Google Scholar] [CrossRef]
- Iattici, F.; Catallo, M.; Solieri, L. Designing new yeasts for craft brewing: When natural biodiversity meets biotechnology. Beverages 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Štulíková, K.; Vrzal, T.; Kubizniaková, P.; Enge, J.; Matoulková, D.; Brányik, T. Spoilage of bottled lager beer contaminated with Saccharomyces cerevisiae var. diastaticus. J. Inst. Brew. 2021, 127, 256–261. [Google Scholar] [CrossRef]
- Meier-Dörnberg, T.; Kory, O.I.; Jacob, F.; Michel, M.; Hutzler, M. Saccharomyces cerevisiae variety diastaticus friend or foe?—spoilage potential and brewing ability of different Saccharomyces cerevisiae variety diastaticus yeast isolates by genetic, phenotypic and physiological characterization. FEMS Yeast Res. 2018, 18, foy023. [Google Scholar] [CrossRef] [PubMed]
- González, S.S.; Barrio, E.; Gafner, J.; Querol, A. Natural hybrids from Saccharomyces cerevisiae, Saccharomyces bayanus and Saccharomyces kudriavzevii in wine fermentations. FEMS Yeast Res. 2006, 6, 1221–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.-V.; Gaillardin, C. Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res. 2005, 5, 471–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.-V.; Lepingle, A.; Gaillardin, C. Molecular typing demonstrates homogeneity of Saccharomyces uvarum strains and reveals the existence of hybrids between S. uvarum and S. cerevisiae, including the S. bayanus type strain CBS 380. Syst. Appl. Microbiol. 2000, 23, 71–85. [Google Scholar] [CrossRef]
- Rainieri, S.; Kodama, Y.; Nakao, Y.; Pulvirenti, A.; Giudici, P. The inheritance of mtDNA in lager brewing strains. FEMS Yeast Res. 2008, 8, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.S.; Naumov, G.I.; Masneuf-Pomarède, I.; Aigle, M.; Dubourdieu, D. Molecular genetic study of introgression between Saccharomyces bayanus and S. cerevisiae. Yeast 2005, 22, 1099–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.D.; Divol, B. Brettanomyces bruxellensis, a survivalist prepared for the wine apocalypse and other beverages. Food Microbiol. 2016, 59, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M. Two decades of “horse sweat” taint and Brettanomyces yeasts in wine: Where do we stand now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.-M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef] [PubMed]
- Serra Colomer, M.; Funch, B.; Forster, J. The raise of Brettanomyces yeast species for beer production. Curr. Opin. Biotechnol. 2019, 56, 30–35. [Google Scholar] [CrossRef]
- Moktaduzzaman, M.; Galafassi, S.; Vigentini, I.; Foschino, R.; Corte, L.; Cardinali, G.; Piškur, J.; Compagno, C. Strain-dependent tolerance to acetic acid in Dekkera bruxellensis. Ann. Microbiol. 2016, 66, 351–359. [Google Scholar] [CrossRef]
- Blomqvist, J.; Eberhard, T.; Schnürer, J.; Passoth, V. Fermentation characteristics of Dekkera bruxellensis strains. Appl. Microbiol. Biotechnol. 2010, 87, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, D.C.; Cajueiro, D.B.B.; Moreno, I.C.P.; Leite, F.C.B.; De Barros Pita, W.; De Morais, M.A., Jr. On the catabolism of amino acids in the yeast Dekkera bruxellensis and the implications for industrial fermentation processes. Yeast 2018, 35, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Joseph, C.L.; Albino, E.; Bisson, L.F. Creation and use of a Brettanomyces aroma wheel. Catal. Discov. Pract. 2017, 1, 12–20. [Google Scholar] [CrossRef]
- Cardello, A.V.; Meiselman, H.L.; Schutz, H.G.; Craig, C.; Given, Z.; Lesher, L.L.; Eicher, S. Measuring emotional responses to foods and food names using questionnaires. Food Qual. Prefer. 2012, 24, 243–250. [Google Scholar] [CrossRef]
- Thomson, D.M.; Crocker, C.; Marketo, C.G. Linking sensory characteristics to emotions: An example using dark chocolate. Food Qual. Prefer. 2010, 21, 1117–1125. [Google Scholar] [CrossRef]
- Gutjar, S.; de Graaf, C.; Kooijman, V.; de Wijk, R.A.; Nys, A.; ter Horst, G.J.; Jager, G. The role of emotions in food choice and liking. Food Res. Int. 2015, 76, 216–223. [Google Scholar] [CrossRef]
- Dalenberg, J.R.; Gutjar, S.; ter Horst, G.J.; de Graaf, K.; Renken, R.J.; Jager, G. Evoked Emotions Predict Food Choice. PLoS ONE 2014, 9, e115388. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, J.; Zandstra, E.H.; Lion, R.; Blanchette, I.; Thomas, A.; El-Deredy, W. Conditioning unfamiliar and familiar flavours to specific positive emotions. Food Qual. Prefer. 2010, 21, 1105–1107. [Google Scholar] [CrossRef]
- Bryan. Fact or Fiction—Biogenic Amines in Beer. Sui Generis Brew. 2019. Available online: https://suigenerisbrewing.com/index.php/2019/01/22/biogenic-amines/ (accessed on 15 June 2022).
- Kalač, P.; Šavel, J.; Křížek, M.; Pelikánová, T.; Prokopová, M. Biogenic amine formation in bottled beer. Food Chem. 2002, 79, 431–434. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255. [Google Scholar] [CrossRef]
- Fernández, M.; del Río, B.; Linares, D.M.; Martín, M.C.; Alvarez, M.A. Real-Time Polymerase Chain Reaction for Quantitative Detection of Histamine-Producing Bacteria: Use in Cheese Production. J. Dairy Sci. 2006, 89, 3763–3769. [Google Scholar] [CrossRef] [Green Version]
- Ladero, V.; Calles-Enriquez, M.; Fernandez, M.; Alvarez, M.A. Toxicological Effects of Dietary Biogenic Amines. Curr. Nutr. Food Sci. 2010, 6, 145–156. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef] [Green Version]
- McCabe-Sellers, B.; Frankel, E.H.; Wolfe, J.J. (Eds.) Handbook of Food-Drug Interactions; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-429-20832-4. [Google Scholar]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B.; et al. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomino-Vasco, M.; Rodríguez-Cáceres, M.I.; Mora-Diez, N.; Pardo-Botello, R.; Acedo-Valenzuela, M.I. Biogenic amines profile in red wines regarding aging and storage conditions. J. Food Compos. Anal. 2019, 83, 103295. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 29 May 2022).
- Ladero, V.; Linares, D.M.; Pérez, M.; del Rio, B.; Fernández, M.; Alvarez, M.A. Biogenic Amines in Dairy Products. In Microbial Toxins in Dairy Products; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 94–131. ISBN 978-1-118-82309-5. [Google Scholar]
- LST 1572:2004 Beer. Determination of Ethyl Alcohol Concentration, True and Starting Extract (LST 1572, 2004 June)/1K:2008/P:2017). 2004. Available online: https://eshop.lsd.lt/public#!/product/list/home?filter=%7B%22page%22:1,%22pageSize%22:50,%22newlyCreated%22:true%7D (accessed on 21 June 2022).
- ISO 4316:1977. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/01/10185.html (accessed on 20 June 2022).
- LST 1990:2007 Beer. Determination of Acidity (LST 1990:2007). 1990. Available online: https://eshop.lsd.lt/public#!/product/list/home?filter=%7B%22page%22:1,%22pageSize%22:50,%22newlyCreated%22:true%7D (accessed on 30 May 2022).
- LST 1490:2006 Beer Wort and Beer. Determination of Color by Spectrophotometric Method (LST 1490, 2006 m. April) 2006. Available online: https://eshop.lsd.lt/public#!/product/list/home?filter=%7B%22page%22:1,%22pageSize%22:50,%22newlyCreated%22:true%7D (accessed on 22 May 2022).
- Philpott, J.; Taylor, D.M.; Williams, D.R. Critical Assessment of Factors Affecting the Accuracy of the IoB Bitterness Method. J. Am. Soc. Brew. Chem. 1997, 55, 103–106. [Google Scholar] [CrossRef]
- ISO 8586-1:1993 Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/58/15875.html (accessed on 15 August 2021).
- Bartkiene, E.; Mockus, E.; Mozuriene, E.; Klementaviciute, J.; Monstaviciute, E.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Cernauskas, D.; Klupsaite, D. The Evaluation of Dark Chocolate-Elicited Emotions and Their Relation with Physico Chemical Attributes of Chocolate. Foods 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gigirey, B.; Vieites Baptista de Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef]
- Lasanta, C.; Durán-Guerrero, E.; Díaz, A.B.; Castro, R. Influence of fermentation temperature and yeast type on the chemical and sensory profile of handcrafted beers. J. Sci. Food Agric. 2021, 101, 1174–1181. [Google Scholar] [CrossRef]
- Kolpin, K.M.; Shellhammer, T.H. The human bitterness detection threshold of iso-α-acids and tetrahydro-iso-α-acids in lager beer. J. Am. Soc. Brew. Chem. 2009, 67, 200–205. [Google Scholar] [CrossRef]
- Cocuzza, S.; Zarnkow, M.; Stallforth, A.; Peifer, F.; Jacob, F. The impact of dry hopping on selected physical and chemical attributes of beer. Brew. Sci. 2019, 72, 118–124. [Google Scholar]
- Łukasz, W.; Marcin, C. What Drives the Development of Craft Beer Production in Poland—Insights from a Hedonic Regression Analysis of the Polish Beer Market. East. Eur. Econ. 2021, 59, 597–610. [Google Scholar] [CrossRef]
- Drewnowski, A. The science and complexity of bitter taste. Nutr. Rev. 2001, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dorado, R.; Chaya, C.; Hort, J. The impact of PROP and thermal taster status on the emotional response to beer. Food Qual. Prefer. 2018, 68, 420–430. [Google Scholar] [CrossRef]
- Yoon, E.; Kim, J.; Lee, J. The US Consumers’ Acceptability and Emotion Measures when Consuming Novel Korean Traditional Non-Alcoholic Beverages. J. Sens. Stud. 2016, 31, 256–271. [Google Scholar] [CrossRef]
- Guinard, J.-X.; Uotani, B.; Mazzucchelli, R.; Taguchi, A.; Masuoka, S.; Fujino, S. Consumer testing of commercial lager beers in blind versus informed conditions: Relation with descriptive analysis and expert quality ratings. J. Inst. Brew. 2000, 106, 11–20. [Google Scholar] [CrossRef]
- Donadini, G.; Fumi, M.D.; Kordialik-Bogacka, E.; Maggi, L.; Lambri, M.; Sckokai, P. Consumer interest in specialty beers in three European markets. Food Res. Int. 2016, 85, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Rousmans, S.; Robin, O.; Dittmar, A.; Vernet-Maury, E. Autonomic nervous system responses associated with primary tastes. Chem. Senses 2000, 25, 709–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blondin, B.; Dequin, S.; Querol, A.; Legras, J.-L. Genome of Saccharomyces cerevisiae and related yeasts. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin, Germany, 2009; pp. 361–378. [Google Scholar]
- Gamero, A.; Belloch, C.; Ibáñez, C.; Querol, A. Molecular Analysis of the Genes Involved in Aroma Synthesis in the Species S. cerevisiae, S. kudriavzevii and S. bayanus var. uvarum in Winemaking Conditions. PLoS ONE 2014, 9, e97626. [Google Scholar] [CrossRef] [PubMed]
- Tosi, E.; Azzolini, M.; Guzzo, F.; Zapparoli, G. Evidence of different fermentation behaviours of two indigenous strains of Saccharomyces cerevisiae and Saccharomyces uvarum isolated from Amarone wine. J. Appl. Microbiol. 2009, 107, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Chemical constituents of grapes and wine. Wine Sci. 2008, 270–331. [Google Scholar]
- Delfini, C.; Gaia, P.; Mariscalco, G.; Contiero, M.; Pagliara, A. Production of benzaldehyde, benzyl alcohol and benzoic acid by yeasts and Botrytis cinerea isolated from grape musts and wines. Vitis 1991, 30, 253–263. [Google Scholar]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2009; Volume 154, p. 196. [Google Scholar]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Kang, W.; Xu, Y.; Qin, L.; Wang, Y. Effects of different β-d-glycosidases on bound aroma compounds in Muscat grape determined by HS-SPME and GC-MS. J. Inst. Brew. 2010, 116, 70–77. [Google Scholar] [CrossRef]
- Nalazek-Rudnicka, K.; Wojnowski, W.; Wasik, A. Occurrence and Levels of Biogenic Amines in Beers Produced by Different Methods. Foods 2021, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- Lorencová, E.; Salek, R.N.; Černíková, M.; Buňková, L.; Hýlková, A.; Buňka, F. Biogenic amines occurrence in beers produced in Czech microbreweries. Food Control 2020, 117, 107335. [Google Scholar] [CrossRef]
- Loret, S.; Deloyer, P.; Dandrifosse, G. Levels of biogenic amines as a measure of the quality of the beer fermentation process: Data from Belgian samples. Food Chem. 2005, 89, 519–525. [Google Scholar] [CrossRef]
- Tang, T.; Shi, T.; Qian, K.; Li, P.; Li, J.; Cao, Y. Determination of biogenic amines in beer with pre-column derivatization by high performance liquid chromatography. J. Chromatogr. B 2009, 877, 507–512. [Google Scholar] [CrossRef]
- Nalazek-Rudnicka, K.; Kubica, P.; Wasik, A. Discrepancies in determination of biogenic amines in beer samples by reversed phase and hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Microchem. J. 2020, 159, 105574. [Google Scholar] [CrossRef]
- Poveda, J.M. Biogenic amines and free amino acids in craft beers from the Spanish market: A statistical approach. Food Control 2019, 96, 227–233. [Google Scholar] [CrossRef]
- Önal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Shalaby, A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996, 29, 675–690. [Google Scholar] [CrossRef]
- Costa, M.P.; Rodrigues, B.L.; Frasao, B.S.; Conte-Junior, C.A. Chapter 2—Biogenic Amines as Food Quality Index and Chemical Risk for Human Consumption. In Hand of Food Bioengineering: Food Quality: Balancing Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 75–108. ISBN 978-0-12-811442-1. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2393 (accessed on 26 July 2022).
- Gardner, D.M.; Shulman, K.I.; Walker, S.E.; Tailor, S.A. The making of a user friendly MAOI diet. J. Clin. Psychiatry 1996, 57, 99–104. [Google Scholar]
- Kalac, P.; Krízek, M. A Review of Biogenic Amines and Polyamines in Beer. J. Inst. Brew. 2003, 109, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Lippman, S.B.; Nash, K. Monoamine oxidase inhibitor update. Potential adverse food and drug interactions. Drug Saf. 1990, 5, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Walker, J.F.; Doyle, J.S. Tyramine in alcohol-free beer. Lancet 1988, 1, 1167–1168. [Google Scholar] [CrossRef]
- Shulman, K.I.; Tailor, S.A.; Walker, S.E.; Gardner, D.M. Tap (draft) beer and monoamine oxidase inhibitor dietary restrictions. Can. J. Psychiatry Rev. Can. Psychiatr. 1997, 42, 310–312. [Google Scholar] [CrossRef]
- Tailor, S.A.N.; Shulman, K.I.; Walker, S.E.; Moss, J.; Gardner, D. Hypertensive episode associated with phenelzine and tap beer: A reanalysis of the role of pressor amines in beer. J. Clin. Psychopharmacol. 1994, 14, 5–14. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Beer Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | |||||||
| Duration of Maturation | |||||||||
| 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | |
| Alcoholic grade (% v/v) | 7.67 ± 0.42 a,B | 7.95 ± 0.36 a,B | 8.14 ± 0.22 a,C | 6.55 ± 0.34 a,A | 6.55 ± 0.43 a,A | 6.56 ± 0.27 a,A | 7.32 ± 0.23 a,B | 7.49 ± 0.34 a,B | 7.65 ± 0.21 a,B |
| Dense materials, Plato % | 15.30 ± 0.40 a,A | 15.27 ± 0.24 a,A | 15.29 ± 0.33 a,A | 15.29 ± 0.35 a,A | 15.28 ± 0.15 a,A | 15.30 ± 0.20 a,A | 15.30 ± 0.21 a,A | 15.28 ± 0.32 a,A | 15.26 ± 0.24 a,A |
| Density, g/cm | 0.990 ± 0.007 a,A | 0.990 ± 0.005 a,A | 0.990 ± 0.006 a,A | 0.988 ± 0.003 a,A | 0.989 ± 0.005 a,A | 0.986 ± 0.004 a,A | 0.989 ± 0.005 a,A | 0.989 ± 0.003 a,A | 0.989 ± 0.002 a,A |
| Density, SG units | 1.00 ± 0.08 a,A | 1.00 ± 0.03 a,A | 1.00 ± 0.07 a,A | 1.01 ± 0.02 a,A | 1.01 ± 0.03 a,A | 1.01 ± 0.04 a,A | 1.01 ± 0.05 a,A | 1.01 ± 0.04 a,A | 1.00 ± 0.06 a,A |
| RDF, % | 76.66 ± 3.43 a,B | 79.47 ± 2.67 a,B | 81.29 ± 4.47 a,B | 65.57 ± 2.75 a,A | 65.58 ± 2.84 a,A | 65.59 ± 3.85 a,A | 73.22 ± 4.4 a,B | 74.90 ± 3.23 a,B | 76.52 ± 3.12 a,B |
| ADF, % | 92.83 ± 4.91 a,B | 96.48 ± 3.82 a,B | 98.85 ± 2.68 a,C | 78.58 ± 3.23 a,A | 78.6 ± 3.36 a,A | 78.61 ± 2.94 a,A | 88.38 ± 2.84 a,B | 90.55 ± 4.98 a,B | 92.65 ± 2.57 a,B |
| pH | 4.40 ± 0.03 a,B | 4.42 ± 0.02 a,B | 4.36 ± 0.04 a,B | 4.44 ± 0.04 a,B | 4.43 ± 0.03 a,B | 4.40 ± 0.02 a,B | 4.09 ± 0.02 a,A | 4.10 ± 0.03 a,A | 4.09 ± 0.01 a,A |
| TA (lactic acid), g/L | 1.00 ± 0.010 c,B | 0.800 ± 0.030 b,B | 0.700 ± 0.040 a,B | 0.400 ± 0.030 a,A | 0.400 ± 0.020 a,A | 0.400 ± 0.020 a,A | 2.10 ± 0.110 c,C | 1.80 ± 0.140 b,C | 1.40 ± 0.130 a,C |
| Colour intensity, EBC | 8.60 ± 0.07 a,A | 10.3 ± 0.19 c,A | 9.70 ± 0.07 b,B | 10.2 ± 0.04 a,B | 10.7 ± 0.05 b,C | 10.2 ± 0.03 a,C | 10.4 ± 0.04 b,C | 10.6 ± 0.05 c,B | 9.30 ± 0.06 a,A |
| Bitterness, BU | 37.3 ± 2.80 a,A | 35.8 ± 2.63 a,A | 33.5 ± 1.93 a,A | 34.2 ± 2.40 a,A | 34.3 ± 2.71 a,A | 34.1 ± 2.08 a,A | 35.7 ± 3.16 a,A | 38.4 ± 2.36 a,A | 35.7 ± 3.49 a,A |
| Energy value, kCal/100 mL | 58.0 ± 1.70 a,A | 58.0 ± 2.50 a,A | 58.0 ± 1.60 a,A | 59.0 ± 2.60 a,A | 59.0 ± 2.30 a,A | 59.0 ± 1.80 a,A | 58.0 ± 1.10 a,A | 58.0 ± 1.40 a,A | 58.0 ± 3.70 a,A |
| Energy value, kJ/100 mL | 241 ± 3.6 a,A | 240 ± 4.1 a,A | 240 ± 5.7 a,A | 243 ± 3.3 a,A | 243 ± 2.4 a,A | 243 ± 3.9 a,A | 241 ± 2.1 a,A | 241 ± 3.6 a,A | 241 ± 2.7 a,A |
| Beer Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | |||||||
| Duration of Maturation | |||||||||
| 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | |
| Overall acceptability from 10 (extremely like) to 1 (extremely dislike) | |||||||||
| 7.4 ±1.5 a,A | 8.1 ± 2.3 a,A | 8.0 ± 1.2 a,B | 8.1 ± 2.0 a,A | 8.4 ± 1.5 a,A | 7.4 ± 1.0 a,B | 6.9 ± 1.8 a,A | 7.7 ± 2.4 a,A | 5.2 ± 1.1 a,A | |
| Emotions induced by the beer (from 0 to 1) | |||||||||
| Neutral | 0.691 ± 0.015 a,A | 0.743 ± 0.016 b,A,B | 0.783 ± 0.036 b,B | 0.686 ± 0.042 a,A | 0.784 ± 0.051 b,B | 0.720 ± 0.029 b,A,B | 0.725 ± 0.020 b,A | 0.677 ± 0.012 a,A | 0.676 ± 0.020 a,A |
| Happy | 0.056 ± 0.010 b,B | 0.034 ± 0.011 a,B | 0.084 ± 0.009 c,B | 0.098 ± 0.011 c,C | 0.017 ± 0.002 a,A | 0.060 ± 0.014 b,A | 0.028 ± 0.004 a,A | 0.049 ± 0.007 b,B | 0.097 ± 0.013 c,B |
| Sad | 0.053 ± 0.008 c,A | 0.034 ± 0.006 b,A | 0.022 ± 0.002 a,A | 0.043 ± 0.004 b,A | 0.033 ± 0.005 a,A | 0.031 ± 0.003 a,B | 0.055 ± 0.008 b,A | 0.036 ± 0.003 a,A | 0.052 ± 0.004 b,C |
| Angry | 0.019 ± 0.003 a,A | 0.035 ± 0.004 b,A | 0.021 ± 0.002 a,A | 0.030 ± 0.004 a,B | 0.032 ± 0.005 a,A | 0.057 ± 0.008 b,C | 0.065 ± 0.009 b,C | 0.039 ± 0.007 a,A | 0.033 ± 0.004 a,B |
| Surprised | 0.025 ± 0.003 c,B | 0.013 ± 0.001 b,A | 0.007 ± 0.002 a,A | 0.019 ± 0.002 b,A | 0.018 ± 0.002 b,B | 0.005 ± 0.003 a,A | 0.018 ± 0.002 c,A | 0.010 ± 0.002 b,A | 0.004 ± 0.003 a,A |
| Scared | 0.016 ± 0.004 b,C | 0.016 ± 0.004 b,B | 0.004 ± 0.002 a,B | 0.004 ± 0.001 a,A | 0.013 ± 0.002 b,B | 0.007 ± 0.003 a,B | 0.008 ± 0.002 b,B | 0.008 ± 0.001 b,A | 0.002 ± 0.001 a,A |
| Disgusted | 0.024 ± 0.005 b,B | 0.014 ± 0.002 a,A | 0.012 ± 0.003 a,A | 0.024 ± 0.005 b,B | 0.012 ± 0.003 a,A | 0.009 ± 0.002 a,A | 0.013 ± 0.002 a,A | 0.021 ± 0.004 b,B | 0.019 ± 0.005 b,A |
| Contempt | 0.007 ± 0.003 a,A | 0.004 ± 0.002 a,A | 0.004 ± 0.002 a,A | 0.011 ± 0.002 b,A | 0.008 ± 0.002 b,A | 0.004 ± 0.001 a,A | 0.013 ± 0.002 b,A,B | 0.004 ± 0.002 a,A | 0.004 ± 0.001 a,A |
| Valence (from −1 to 1) | |||||||||
| −0.034 ± 0.007 b,B | −0.055 ± 0.009 a,A | 0.007 ± 0.003 c,B | 0.012 ± 0.006 c,C | −0.057 ± 0.007 a,A | −0.023 ± 0.008 b,A | −0.093 ± 0.012 a,A | −0.034 ± 0.006 b,B | 0.019 ± 0.007 c,C | |
| Factor | Dependent Variable | p |
|---|---|---|
| Yeast strain used for fermentation | OA | 0.225 |
| neutral | 0.0001 | |
| happy | 0.998 | |
| sad | 0.0001 | |
| angry | 0.0001 | |
| surprised | 0.0001 | |
| Duration of maturation | OA | 0.377 |
| neutral | 0.001 | |
| happy | 0.0001 | |
| sad | 0.0001 | |
| angry | 0.579 | |
| surprised | 0.0001 | |
| Yeast strain used for fermentation * Duration of maturation | OA | 0.767 |
| neutral | 0.0001 | |
| happy | 0.0001 | |
| sad | 0.0001 | |
| angry | 0.0001 | |
| surprised | 0.0001 |
| VC, % from the Total VC Content | Beer Samples | Correlations (r) and Their Significance (p) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | between OA and VC | between ‘H’ and VC | ||||||||
| Duration of Maturation | ||||||||||||
| 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | ||||
| 3-methyl-1-butanol | 10.52 a,B | 18.0 b,B | 20.56 b,B | 14.41 a,C | 15.69 a,A | 21.67 b,B | 8.47 a,A | 14.95 b,A | 17.11 b,A | r | 0.441 * | 0.317 |
| p | 0.021 | 0.108 | ||||||||||
| Styrene | 4.26 a,B | 6.13 a,B | 5.64 a,B | 9.25 a,B | 11.89 a,C | 11.97 a,C | 1.93 b,A | 3.28 c,A | 0.992 a,A | r | 0.131 | 0.067 |
| p | 0.515 | 0.740 | ||||||||||
| β-myrcene | 1.14 b,A | 1.06 b,A | 0.718 a,A | 1.43 b,A | 1.38 b,A | 0.820 a,B | 1.47 b,A | 1.42 b,A | 0.911 a,C | r | −0.193 | 0.575 ** |
| p | 0.336 | 0.002 | ||||||||||
| Hexanoic acid, ethyl ester | 1.10 a,A | 1.41 a,B | 1.26 a,B | 2.33 a,B | 3.08 b,C | 3.91 b,C | 0.931 b,A | 0.599 a,A | 0.541 a,A | r | −0.130 | −0.197 |
| p | 0.517 | 0.324 | ||||||||||
| 2-Methylbutylbutanoate | 2.82 a,B | 2.89 a,A | 3.06 a,A,B | 1.91 a,A | 2.82 b,A | 3.95 c,B | 2.86 a,B | 2.82 a,A | 2.49 a,A | r | 0.401 * | −0.228 |
| p | 0.038 | 0.252 | ||||||||||
| L-α-Terpineol | 24.7 a,A | 26.9 a,C | 23.6 a,B | 19.6 b,A | 20.4 b,A | 16.9 a,A | 22.3 a,A | 24.5 a,B | 24.7 a,B | r | 0.319 | 0.034 |
| p | 0.104 | 0.867 | ||||||||||
| Decanal | 1.04 b,B | 0.353 a,A | 0.476 a,A | 0.513 a,A | 0.969 b,C | 0.857 b,B | 1.28 b,B | 0.582 a,B | 0.375 a,A | r | 0.354 | −0.068 |
| p | 0.070 | 0.736 | ||||||||||
| Acetic acid, octyl ester | 0.682 b,A | 0.430 a,A | 0.357 a,A | 0.750 b,B | 0.607 b,B | 0.357 a,A | 0.986 c,C | 0.682 b,B | 0.504 a,B | r | 0.178 | −0.183 |
| p | 0.373 | 0.360 | ||||||||||
| Hexanoic acid 3-methylbutyl ester | 0.702 a,B | 0.705 a,B | 0.803 b,B | 0.411 a,A | 0.377 a,A | 0.355 a,A | 1.81 b,C | 0.796 a,B | 1.28 b,C | r | 0.403 * | 0.052 |
| p | 0.037 | 0.797 | ||||||||||
| Hexanoic acid 2-methylbutyl ester | 21.2 b,A | 15.2 a,A | 15.6 a,B | 22.5 A | 16.7 b,A | 10.4 a,A | 27.1 b,B | 19.9 a,B | 20.6 a,C | r | 0.049 | −0.297 |
| p | 0.809 | 0.132 | ||||||||||
| Acetic acid, 2-phenylethyl ester | 3.59 a,B | 4.75 b,B | 4.53 b,B | 2.32 a,A | 2.88 a,A | 4.76 b,B | 3.21 a,B | 4.05 b,B | 3.38 a,A | r | 0.259 | −0.076 |
| p | 0.191 | 0.706 | ||||||||||
| Ethyl trans-4-decenoate | 1.50 a,B | 1.68 a,B | 1.85 a,B | 1.47 a,B | 1.67 a,B | 2.31 b,C | 0.531 a,A | 0.739 b,A | 0.715 b,A | r | −0.459 * | 0.131 |
| p | 0.016 | 0.516 | ||||||||||
| Ethyl 9-decenoate | 1.21 a,A | 1.70 b,A | 1.79 b,A | 1.84 a,B | 2.24 a,B | 2.08 a,B | 1.64 a,A,B | 2.25 b,B | 2.31 b,B | r | 0.101 | 0.223 |
| p | 0.616 | 0.264 | ||||||||||
| Caryophyllene | nd | 0.375 a | 0.644 b,A | nd | nd | 0.740 A | nd | nd | 1.06 B | r | 0.184 | 0.171 |
| p | 0.358 | 0.394 | ||||||||||
| α-humulene | nd | nd | nd | nd | nd | nd | nd | 1.48 a | 2.43 b | r | −0.344 | 0.382 * |
| p | 0.079 | 0.049 | ||||||||||
| n-Capric acid isobutyl ester | 10.3 b,B | 4.94 a,A | 4.24 a,A | 6.17 b,A | 4.97 a,A | 4.33 a,A | 11.1 b,B | 8.57 a,B | 7.28 a,B | r | −0.073 | −0.047 |
| p | 0.717 | 0.815 | ||||||||||
| Ethyl dodecanoate | 5.91 a,C | 7.75 b,C | 8.76 c,C | 3.89 a,B | 4.71 b,A | 6.21 c,B | 2.91 a,A | 3.95 b,A | 4.75 c,A | r | 0.308 | 0.167 |
| p | 0.119 | 0.404 | ||||||||||
| Dependent Variable | Significance (p) of the Influence of Factor and Factors Interaction | ||
|---|---|---|---|
| Yeast Strain Used for Fermentation | Duration of Maturation | Yeast Strain Used for Fermentation * Duration of Maturation | |
| 3-methyl-1-butanol | 0.156 | 0.034 | 0.539 |
| Styrene | 0.001 | 0.0001 | 0.010 |
| β-Myrcene | 0.256 | 0.0001 | 0.455 |
| Hexanoic acid, ethyl ester | 0.0001 | 0.107 | 0.0001 |
| 2-Methylbutylbutanoate | 0.974 | 0.307 | 0.023 |
| L-α-Terpineol | 0.375 | 0.619 | 0.637 |
| Decanal | 0.0001 | 0.0001 | 0.062 |
| Acetic acid, octyl ester | 0.054 | 0.007 | 0.505 |
| Hexanoic acid 3-methylbutyl ester | 0.0001 | 0.003 | 0.0001 |
| Hexanoic acid 2-methylbutyl ester | 0.002 | 0.0001 | 0.337 |
| Acetic acid, 2-phenylethyl ester | 0.010 | 0.152 | 0.886 |
| Ethyl trans-4-decenoate | 0.0001 | 0.089 | 0.722 |
| Ethyl 9-decenoate | 0.0001 | 0.009 | 0.003 |
| Caryophyllene | 0.029 | 0.001 | 0.093 |
| α-humulene | 0.0001 | 0.0001 | 0.0001 |
| n-Capric acid isobutyl ester | 0.0001 | 0.0001 | 0.047 |
| Ethyl dodecanoate | 0.0001 | 0.0001 | 0.121 |
| Biogenic Amines Concentration, mg/kg | Beer Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | |||||||
| Duration of Maturation | |||||||||
| 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | 1 Day | 30 Days | 90 Days | |
| Tryptamine | 42.95 ± 5.63 a,A | 75.34 ± 8.62 b,A | nd | 60.56 ± 9.37 a,B | 66.46 ± 7.03 a,A | nd | 60.92 ± 7.21 a,B | 69.51 ± 5.63 b,A | nd |
| Phenylethylamine | 18.51 ± 1.29 a,A | 24.46 ± 3.41 b,A | 35.37 ± 3.72 c,A | 22.99 ± 4.54 a,A,B | 30.54 ± 2.15 b,B | 36.95 ± 5.02 b,A | 23.13 ± 2.07 a,A | 29.31 ± 1.29 b,A,B | 57.73 ± 5.41 c, B |
| Putrescine | nd | nd | 17.58 ± 2.9 A | nd | 22.27 ± 2.51 a,A | 20.12 ± 2.13 a,A | nd | 348.93 ± 9.78 b,B | 305.1 ± 8.52 a,B |
| Cadaverine | nd | nd | nd | nd | nd | nd | nd | 223.8 ± 14.2 b | 182.5 ± 11.3 a |
| Histamine | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Tyramine | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Spermidine | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Spermine | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Pearson Correlation and Significance | Tryptamine | Phenylethylamine | Putrescine | Cadaverine | |
|---|---|---|---|---|---|
| Overall acceptability | Pearson Correlation | 0.382 * | 0.028 | −0.269 | −0.252 |
| Sig. (2-tailed) | 0.049 | 0.890 | 0.175 | 0.205 | |
| Emotion “neutral” | Pearson Correlation | 0.021 | 0.151 | −0.515 ** | −0.545 ** |
| Sig. (2-tailed) | 0.916 | 0.453 | 0.006 | 0.003 | |
| Emotion “happy” | Pearson Correlation | −0.552 ** | 0.361 | 0.241 | 0.233 |
| Sig. (2-tailed) | 0.003 | 0.065 | 0.226 | 0.242 | |
| Emotion “sad” | Pearson Correlation | 0.205 | −0.264 | 0.124 | 0.156 |
| Sig. (2-tailed) | 0.304 | 0.183 | 0.538 | 0.439 | |
| Emotion “angry” | Pearson Correlation | 0.100 | 0.025 | −0.015 | −0.008 |
| Sig. (2-tailed) | 0.620 | 0.903 | 0.940 | 0.967 | |
| Emotion “surprised” | Pearson Correlation | 0.647 ** | −0.588 ** | −0.501 ** | −0.459 * |
| Sig. (2-tailed) | 0.0001 | 0.001 | 0.008 | 0.016 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matukas, M.; Starkute, V.; Zokaityte, E.; Zokaityte, G.; Klupsaite, D.; Mockus, E.; Rocha, J.M.; Ruibys, R.; Bartkiene, E. Effect of Different Yeast Strains on Biogenic Amines, Volatile Compounds and Sensory Profile of Beer. Foods 2022, 11, 2317. https://doi.org/10.3390/foods11152317
Matukas M, Starkute V, Zokaityte E, Zokaityte G, Klupsaite D, Mockus E, Rocha JM, Ruibys R, Bartkiene E. Effect of Different Yeast Strains on Biogenic Amines, Volatile Compounds and Sensory Profile of Beer. Foods. 2022; 11(15):2317. https://doi.org/10.3390/foods11152317
Chicago/Turabian StyleMatukas, Mazvydas, Vytaute Starkute, Egle Zokaityte, Gintare Zokaityte, Dovile Klupsaite, Ernestas Mockus, João Miguel Rocha, Romas Ruibys, and Elena Bartkiene. 2022. "Effect of Different Yeast Strains on Biogenic Amines, Volatile Compounds and Sensory Profile of Beer" Foods 11, no. 15: 2317. https://doi.org/10.3390/foods11152317