In Vitro Hypoglycemic and Anti-Inflammatory Potential and Toxicity of Powders from Pulp and by-Products of Ziziphus mistol from Argentina
Abstract
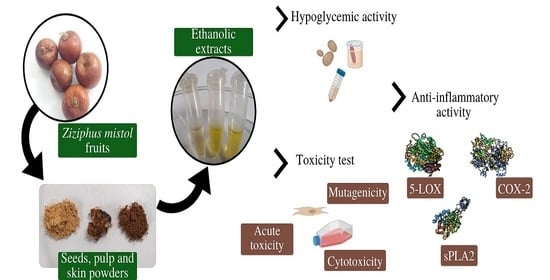
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Powders
2.2. Polyphenols-Enriched Extracts
2.3. Simulated Gastroduodenal Digestion of Mistol Powder and PEE
2.4. Hypoglycemic Activity
2.4.1. Glucose Adsorption of Mistol Powders
2.4.2. Mistol Powders’ Effect on Glucose Diffusion
2.4.3. Improvement in Glucose Uptake in Saccharomyces cerevisiae Cells by Mistol PEE
2.5. Anti-Inflammatory Activity of Mistol PEE
2.5.1. Inhibition of the Enzyme Phospholipase A2 (sPLA2)
2.5.2. Inhibition of the Lipooxygenase Enzyme (LOX)
2.5.3. Inhibition of the Cyclooxygenase Enzyme (COX)
2.6. Toxicity Assays
2.6.1. Acute Toxicity
2.6.2. Salmonella Mutagenicity Assay
2.6.3. Cytotoxicity Tests
2.7. Statistical Analysis
3. Results and Discussion
3.1. Hypoglycemic Effect of Mistol Extracts and Powders
3.1.1. Glucose Adsorption Capacity of Mistol Powders
3.1.2. Effect of Mistol Powders on Glucose Diffusion
3.1.3. Effect of Mistol Powders on Glucose Uptake by Yeast Cells
3.2. Antiinflamatory Activity of Mistol PEE
3.3. Toxicity of Mistol PEE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedictto, M.N.; Valencia, B.G.; Torrella, S.A. Structural and functional characterization of the dry forest in central Argentine Chaco. Madera Bosques 2019, 25, 18. [Google Scholar] [CrossRef]
- Islam, M.B.; Simmons, M.P. A thorny dilemma: Testing alternative intrageneric classifications within Ziziphus (Rhamnaceae). Syst. Bot. 2006, 31, 826–842. [Google Scholar] [CrossRef]
- Cerino, M.C.; Richard, G.A.; Torretta, J.P.; Gutiérrez, H.F.; Pensiero, J.F. Reproductive biology of Ziziphus mistol Griseb. (Rhamnaceae), a wild fruit tree of saline environments. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 211, 18–25. [Google Scholar] [CrossRef]
- Alvarez, M.A. Chaco and Espinal. In Pharmacological Properties of Native Plants from Argentina; Springer: Berlin/Heidelberg, Germany, 2019; pp. 193–226. [Google Scholar]
- Digilio, A.P.; Legname, P.R. Los árboles indígenas de la Provincia de Tucumán. Opera Lilloana 1966, 15, 73. [Google Scholar]
- Matteucci, S.D.; Totino, M.; Arístide, P. Ecological and social consequences of the Forest Transition Theory as applied to the Argentinean Great Chaco. Land Use Policy 2016, 51, 8–17. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species Version 2020–1; IUCN: Gland, Switzerland, 2020. [Google Scholar]
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Alberto, M.R.; Ramos, L.L.P.; Schmeda-Hirschmann, G.; Isla, M.I. Chemical and functional characterization of skin, pulp and seed powder from the Argentine native fruit mistol (Ziziphus mistol). Effects of phenolic fractions on key enzymes involved in metabolic syndrome and oxidative stress. J. Funct. Foods 2017, 37, 531–540. [Google Scholar] [CrossRef]
- Goldfine, A.B.; Shoelson, S.E. Therapeutic approaches targeting inflammation for diabetes and associated cardiovascular risk. J. Clin. Investig. 2017, 127, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Bao, W.; Liu, J.; OuYang, Y.Y.; Wang, D.; Rong, S.; Liu, L.G. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Domingueti, C.P.; Dusse, L.M.S.A.; das Graças Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- WFO. Ziziphus mistol Griseb. 2022. Available online: http://www.worldfloraonline.org/taxon/wfo-0001130914 (accessed on 18 June 2022).
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin- Ciocalteu reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Torres, S.; Zampini, I.C.; Cattaneo, F.; Fernandez Di Pardo, A.; Valle, E.M.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Isla, M.I. Integral use of Argentinean Solanum betaceum red fruits as functional food ingredient to prevent metabolic syndrome: Effect of in vitro simulated gastroduodenal digestion. Heliyon 2020, 6, e03387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Almeida, R.E.; Isla, M.I.; De L Vildoza, E.; Quispe, C.; Schmeda-Hirschmann, G.; Alberto, M.R. Inhibition of arachidonic acid metabolism by the Andean crude drug Parastrephia lucida (Meyen) Cabrera. J. Ethnopharmacol. 2013, 150, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.M.; Mathiasson, L.; Martensson, L.; Bergatröm, S. Artemia salina as test organism for assessment of acute toxicity of leachate water from landfills. Environ. Monit. Assess. 2005, 102, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Carabajal, A.; Piloto-Ferrer, J.; Nicollela, H.; Squarisi, I.; Prado Guissone, A.; Esperandim, T.; Zampini, I.C. Antigenotoxic, antiproliferative and antimetastatic properties of a combination of native medicinal plants from Argentina. J. Ethnopharmacol. 2020, 267, 113479. [Google Scholar] [CrossRef] [PubMed]
- Moradi-Marjaneh, R.; Paseban, M.; Sahebkar, A. Natural products with SGLT2 inhibitory activity: Possibilities of application for the treatment of diabetes. Phytother. Res. 2019, 33, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Benítez, B.; Mollá, E.; Martín-Cabrejas, M.; Aguilera, Y.; Esteban, R. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J. Funct. Foods 2017, 36, 34–42. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Masamba, K.G.; Shoemaker, C.F.; Zhong, F.; Majeed, H.; Ma, J. The effect of chemical treatment on the in vitro hypoglycemic properties of rice bran insoluble dietary fiber. Food Hydrocoll. 2016, 52, 699–706. [Google Scholar] [CrossRef]
- Kabir, A.U.; Samad, M.B.; D’Costa, N.M.; Akhter, F.; Ahmed, A.; Hannan, J.M.A. Anti-hyperglycemic activity of Centella asiatica is partly mediated by carbohydrase inhibition and glucose-fiber binding. BMC Compl. Altern. Med. 2014, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Sairam, S.; Urooj, A. In vitro hypoglycemic effects of selected dietary fiber sources. J. Food Sci. Technol. 2015, 48, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, X.; Ren, D.; Wang, D.; Xuan, Y. Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice. Food Funct. 2014, 5, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, S.; Rajeswari, V.D. Exploring the antidiabetic and anti-obesity properties of Samanea saman through in vitro and in vivo approaches. J. Cell Biochem. 2019, 120, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.T. Small Molecule Kaempferol, a Novel Regulator of Glucose Homeostasis. Doctor of Phylosophy in Human Nutrition, Foods, and Exercise, Faculty of Virginia Polytechnic Institute and State University. 2017. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/80946/Moore_WT_D_2017.pdf?sequence=1&isAllowed=y (accessed on 3 March 2021).
- Alkhalidy, H.; Moore, W.; Wang, A.; Luo, J.; McMillan, R.P.; Wang, Y.; Liu, D. Kaempferol ameliorates hyperglycemia through suppressing hepatic gluconeogenesis and enhancing hepatic insulin sensitivity in diet-induced obese mice. J. Nutr. Biochem. 2018, 58, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Mousinho, N.M.; van Tonder, J.J.; Steenkamp, V. In vitro anti-diabetic activity of Sclerocarya birrea and Ziziphus Mucronata. Nat. Prod. Commun. 2013, 8, 1279–1284. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S. Role of nonsteroidal anti-inflammatory drugs (NSAIDs) in cancer prevention and cancer promotion. Adv. Pharmacol. Sci. 2019, 2019, 3418975. [Google Scholar] [CrossRef] [Green Version]
- Torres, S.; Fabersani, E.; Marquez, A.; Gauffin-Cano, P. Adipose tissue inflammation and metabolic syndrome. The proactive role of probiotics. Eur. J. Nutr. 2019, 58, 27–43. [Google Scholar] [CrossRef]
- Cardozo, M.L.; Ordoñez, R.M.; Alberto, M.R.; Zampini, I.C.; Isla, M.I. Antioxidant and anti-inflammatory activity characterization and genotoxicity evaluation of Ziziphus mistol ripe berries, exotic Argentinean fruit. Food Res. Int. 2011, 44, 2063–2071. [Google Scholar] [CrossRef]
- Pérez, M.J.; Cuello, A.S.; Zampini, I.C.; Ordoñez, R.M.; Alberto, M.R.; Quispe, C.; Isla, M.I. Polyphenolic compounds and anthocyanin content of Prosopis nigra and Prosopis alba pods flour and their antioxidant and anti-inflammatory capacities. Food Res. Int. 2014, 64, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Tran, H.N.K.; Cao, T.Q.; Kim, J.A.; Woo, M.H.; Min, B.S. Anti-Inflammatory and cytotoxic activities of constituents isolated from the fruits of Ziziphus jujuba var. inermis Rehder. Fitoterapia 2019, 137, 104261. [Google Scholar] [CrossRef]
- Borgi, W.; Recio, M.C.; Ríos, J.L.; Chouchane, N. Anti-Inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. S. Afr. J. Bot. 2008, 74, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Kadioglu, O.; Jacob, S.; Bohnert, S.; Naß, J.; Saeed, M.E.; Khalid, H.; Efferth, T. Evaluating ancient Egyptian prescriptions today: Anti-Inflammatory activity of Ziziphus spina-christi. Phytomedicine 2016, 23, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Courts, F.L.; Williamson, G. The occurrence, fate and biological activities of C-glycosyl flavonoids in the human diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the flavonoid C-glycosides and health benefits. Crit. Rev. Food Sci. Nutr. 2015, 56, S29–S45. [Google Scholar] [CrossRef]
- Rashid, H.; Yiming, X.; Ahmad, N.; Muhammad, Y.; Wang, L. Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E2, inducible NO synthase and nuclear factor κB activities. Bioorg. Chem. 2019, 87, 335–365. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Werz, O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol. Adv. 2018, 36, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.S.; Ramanan, M.; Paragi-Vedanthi, P.; Doble, M. Phytochemicals as multi-target inhibitors of the inflammatory pathway-A modeling and experimental study. Biochem. Biophys. Res. Commun. 2017, 484, 467–473. [Google Scholar] [CrossRef]
- Etebari, M.; Zolfaghari, B.; Jafarian-Dehkordi, A.; Mirzaei, A. Hydroalcoholic and polyphenolic extracts of Ziziphus jujuba Mill fruits prevent methyl methanesulfonate-induced DNA damage in HepG2 cells. PRB 2015, 1, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Kaeidi, A.; Taati, M.; Hajializadeh, Z.; Jahandari, F.; Rashidipour, M. Aqueous extract of Zizyphus jujuba fruit attenuates glucose induced neurotoxicity in an in vitro model of diabetic neuropathy. Iran. J. Basic Med. Sci. 2015, 18, 301. [Google Scholar]
- Goswami, P.; Banerjee, R.; Mukherjee, A. Potential antigenotoxicity assessment of Ziziphus jujuba fruit. Heliyon 2019, 5, e01768. [Google Scholar] [CrossRef] [Green Version]
- Rajeh, M.A.B.; Kwan, Y.P.; Zakaria, Z.; Latha, L.Y.; Jothy, S.L.; Sasidharan, S. Acute toxicity impacts of Euphorbia hirta L extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina. Pharmacogn. Res. 2012, 4, 170. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.; Kaleem, W.A.; Qayum, M.; Marwat, I.K.; Zia-Ul-Haq, M.; Ali, I.; Choudhary, M.I. Biological screening of Zizyphus oxyphylla Edgew stem. Pak. J. Bot. 2011, 43, 311–317. [Google Scholar]
- Al-Saeedi, A.H.; Al-Ghafri, M.T.H.; Hossain, M.A. Brine shrimp toxicity of various polarities leaves and fruits crude fractions of Ziziphus jujuba native to Oman and their antimicrobial potency. Sustain. Chem. Pharm. 2017, 5, 122–126. [Google Scholar] [CrossRef]
- Muñoz, S.; Piegari, M.; Guzman, C.; Reynard, A. Differential effects of dietary Oenothera, Zizyphus mistol, and corn oils, and essential fatty acid deficiency on the progression of a murine mammary gland adenocarcinoma. Nutrition 1999, 15, 208–212. [Google Scholar] [CrossRef]




| Sample | Inhibition (%) (100 μg GAE/mL) | ||
|---|---|---|---|
| sPLA2 | LOX | COX-2 | |
| Seeds | NI | 89.8 ± 2.3 a | 91.0 ± 0.9 c |
| Pulp | 46.0 ± 1.2 b | 83.0 ± 1.0 b | 96.0 ± 2.6 b |
| Skin | 25.0 ± 0.6 c | 65.1 ± 0.6 c | 68.5 ± 1.5 d |
| Naproxen | 95.00 ± 2.80 a | - | - |
| Indomethacin | - | 16.00 ± 0.80 d | |
| Nimesulide | - | - | 100.0 ± 5.0 a |
| Sample | μg GAE/Plate | N° Revertants/Plate | MR | ||
|---|---|---|---|---|---|
| TA98 | TA100 | TA98 | TA100 | ||
| Seeds | 175 | 104 ± 17 b | 30 ± 7 b | 0.79 | 1.00 |
| 250 | 110 ± 5 b | 35 ± 2 b | 0.83 | 1.16 | |
| 500 | 110 ± 1 b | 45 ± 4 b | 0.83 | 1.50 | |
| Pulp | 175 | 100 ± 5 b | 16 ± 2 b | 0.76 | 0.53 |
| 250 | 112 ± 12 b | 13 ± 1 b | 0.85 | 0.43 | |
| 500 | 121 ± 20 b | 20 ± 3 b | 0.92 | 0.64 | |
| Skin | 175 | 99 ± 13 b | 19 ± 2 b | 0.75 | 0.63 |
| 250 | 112 ± 5 b | 25 ± 5 b | 0.85 | 0.83 | |
| 500 | 136 ± 11 b | 28 ± 2 b | 1.03 | 0.93 | |
| Negative control (DMSO) | 131 ± 37 b | 30 ± 6 b | |||
| Positive control (4-NPD) | 2861 ± 114 a | 1998 ± 38 a | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orqueda, M.E.; Torres, S.; Zampini, I.C.; Isla, M.I. In Vitro Hypoglycemic and Anti-Inflammatory Potential and Toxicity of Powders from Pulp and by-Products of Ziziphus mistol from Argentina. Foods 2022, 11, 2125. https://doi.org/10.3390/foods11142125
Orqueda ME, Torres S, Zampini IC, Isla MI. In Vitro Hypoglycemic and Anti-Inflammatory Potential and Toxicity of Powders from Pulp and by-Products of Ziziphus mistol from Argentina. Foods. 2022; 11(14):2125. https://doi.org/10.3390/foods11142125
Chicago/Turabian StyleOrqueda, María Eugenia, Sebastian Torres, Iris Catiana Zampini, and María Inés Isla. 2022. "In Vitro Hypoglycemic and Anti-Inflammatory Potential and Toxicity of Powders from Pulp and by-Products of Ziziphus mistol from Argentina" Foods 11, no. 14: 2125. https://doi.org/10.3390/foods11142125