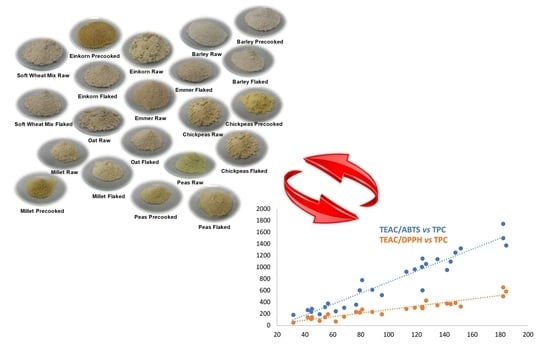
Effect of Flaking and Precooking Procedures on Antioxidant Potential of Selected Ancient Cereal and Legume Flours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Analytical Methods
2.3.1. Starch and Crude Proteins Determination
2.3.2. Extraction of Antioxidant Compounds
2.3.3. Antioxidant Compounds and Pigments Characterization
2.4. Statistical Analysis
3. Results and Discussion
3.1. Starch and Crude Proteins Determination
3.2. Free and Bound Polyphenols and Flavonoids Contents
3.3. Antioxidant Capacity by the ABTS and DPPH Assays
3.4. Carotenoid Content and Antioxidant Activity Quantification by α-TEAC in Lipophilic Extracts
3.5. Chlorophylls Determination in Pea Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Giambanelli, E.; Ferioli, F.; Koçaoglu, B.; Jorjadze, M.; Alexieva, I.; Darbinyan, N.; D’Antuono, L.F. A comparative study of bioactive compounds in primitive wheat populations from Italy, Turkey, Georgia, Bulgaria and Armenia. J. Sci. Food Agric. 2013, 93, 3490–3501. [Google Scholar] [CrossRef]
- Monnet, A.F.; Laleg, K.; Michon, C.; Micard, V. Legume enriched cereal products: A generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019, 86, 131–143. [Google Scholar] [CrossRef]
- Consumi, M.; Tamasi, G.; Pepi, S.; Leone, G.; Bonechi, C.; Magnani, A.; Donati, A.; Rossi, C. Analytical composition of flours through thermogravimetric and rheological combined methods. Thermochim. Acta 2022, 711, 179204. [Google Scholar] [CrossRef]
- Flight, I.; Clifton, P. Cereal grains and legumes in the prevention of coronary heart disease and stroke: A review of the literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Consumi, M.; Leone, G.; Pepi, S.; Pardini, A.; Lamponi, S.; Bonechi, C.; Tamasi, G.; Rossi, C.; Magnani, A. Calcium ions hyaluronan/gellan gum protective shell for delivery of oleuropein in the knee. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 414–424. [Google Scholar] [CrossRef]
- Tullio, V.; Gasperi, V.; Catani, M.V.; Savini, I. The impact of whole grain intake on gastrointestinal tumors: A focus on colorectal, gastric, and esophageal cancers. Nutrients 2021, 13, 81. [Google Scholar] [CrossRef]
- Marcolongo, P.; Gamberucci, A.; Tamasi, G.; Pardini, A.; Bonechi, C.; Rossi, C.; Giunti, R.; Barone, V.; Borghini, A.; Fiorenzani, P.; et al. Chemical characterisation and antihypertensive effects of locular gel and serum of Lycopersicum esculentum L. var. “Camone” tomato in spontaneously hypertensive rats. Molecules 2020, 25, 25163758. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef]
- Tamasi, G.; Bonechi, C.; Donati, A.; Leone, G.; Rossi, C.; Cini, R.; Magnani, A. Analytical and structural investigation via infrared spectroscopy and Density Functional Methods of cuprous complexes of the antioxidant tripeptide glutathione (GSH). Synthesis and characterization of a novel CuI-GSH compound. Inorg. Chim. Acta 2018, 470, 158–171. [Google Scholar] [CrossRef]
- Foschia, M.; Horstmann, S.W.; Arendt, E.K.; Zannini, E. Legumes as functional ingredients in gluten-free bakery and pasta products. Ann. Rev. Food Sci. Technol. 2017, 8, 75–96. [Google Scholar]
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes-A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Vaios, M.I.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Cereals & Grains Association. Total carotenoid content of cereal grains and flours. In AACC Approved Methods of Analysis, 11th ed.; Method 14-60.01; Cereals & Grains Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Kim, K.H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Schweiggert, R.M.; Würschum, T.; Longin, C.F.H.; Carle, R. Lipophilic antioxidants in wheat (Triticum spp.): A target for breeding new varieties for future functional cereal products. J. Funct. Foods 2016, 20, 594–605. [Google Scholar] [CrossRef]
- Beleggia, R.; Platani, C.; Nigro, F.; Cattivelli, L. A micro-method for the determination of yellow pigment content in durum wheat. J. Cereal Sci. 2010, 52, 106–110. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol. 1999, 3, 152–178. [Google Scholar]
- Tamasi, G.; Baratto, M.C.; Bonechi, C.; Byelyakova, A.; Pardini, A.; Donati, A.; Leone, G.; Consumi, M.; Lamponi, S.; Magnani, A.; et al. Chemical characterization and antioxidant properties of products and by-products from Olea europaea L. Food Sci. Nutr. 2019, 7, 2907–2920. [Google Scholar] [CrossRef] [Green Version]
- Tamasi, G.; Bonechi, C.; Leone, G.; Andreassi, M.; Consumi, M.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Varietal and geographical origin characterization of peaches and nectarines by combining analytical techniques and statistical approach. Molecules 2021, 26, 4128. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Vernon, L.P. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal. Chem. 1960, 32, 1144–1150. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Marina, M.L.; Plaza, M. Strategies for the extraction and analysis of non-extractable polyphenols from plants. J. Chromat. A 2017, 1514, 1–15. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion cooking on functional properties and in vitro starch digestibility of barley-based extrudates from fruit and vegetable by-products. J. Food Sci. 2009, 74, E77–E86. [Google Scholar] [CrossRef]
- Marinelli, V.; Padalino, L.; Conte, A.; Del Nobile, M.A.; Briviba, K. Red grape marc flour as food ingredient in durum wheat spaghetti: Nutritional evaluation and bioaccessibility of bioactive compounds. Food Sci. Technol. Res. 2018, 24, 1093–1100. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The profile and bioaccessibility of phenolic compounds in cereals influenced by improved extrusion cooking treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef]
- Sharma, P.; Gujral, H.S.; Singh, B. Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 2012, 131, 1406–1413. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Brijesh Kumar, T. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Hole, A.S.; Kjos, N.P.; Grimmer, S.; Kohler, A.; Lea, P.; Rasmussen, B.; Lima, L.R.; Narvhus, J.; Sahlstrøm, S. Extrusion of barley and oat improves the bioaccessibility of dietary phenolic acids in growing pigs. J. Agric. Food Chem. 2013, 61, 2739–2747. [Google Scholar] [CrossRef]
- Zielinski, H.; Kozlowska, H.; Lewczuk, B. Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov. Food Sci. Emerg. Technol. 2001, 2, 159–169. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT-Food Sci. Technol. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2012, 133, 1–9. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Pardini, A.; Consumi, M.; Leone, G.; Bonechi, C.; Tamasi, G.; Sangiorgio, P.; Verardi, A.; Rossi, C.; Magnani, A. Effect of different post-harvest storage conditions and heat treatment on tomatine content in commercial varieties of green tomatoes. J. Food Comp. Anal. 2021, 96, 103735. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Bunzel, M.; Ralph, J.; Marita, J.M.; Hatfield, R.D.; Steinhart, H. Diferulates as structural components in soluble and insoluble cereal dietary fibre. J. Sci. Food Agric. 2001, 81, 653–660. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, Y.; Sasaki, M.; Takahashi, N.; Kamoshita, M.; Miyake, S.; Tsubota, K. Neuroprotective effects of lutein in the retina. Curr. Pharm. Des. 2012, 18, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef]
- Zieliński, H.; Michalska, A.; Piskuła, M.K.; Kozłowska, H. Antioxidants in thermally treated buckwheat groats. Mol. Nutr. Food Res. 2006, 50, 824–832. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Dimberg, L.; Kamal-Eldin, A. Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.). J. Agric. Food Chem. 2002, 50, 1890–1896. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Gazza, L. Influence of steaming treatment on chemical and technological characteristics of einkorn (Triticum monococcum L. ssp. monococcum) wholemeal flour. Food Chem. 2008, 111, 549–555. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Paznocht, L.; Kotíková, Z.; Šulc, M.; Lachman, J.; Orsák, M.; Eliášová, M.; Martinek, P. Free and esterified carotenoids in pigented wheat, tritordeum and barley grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Diapari, M.; Jha, A.B.; Tar’an, B.; Arganosa, G.; Warkentin, T.D. Genetic diversity of nutritionally important carotenoids in 94 pea and 121 chickpea accessions. J. Food Comp. Anal. 2015, 43, 49–60. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]


| Flour from Raw Materials | Flour from Flaked Materials | Flour from Precooked Materials |
|---|---|---|
| Cereals | ||
| Soft wheat mix 1 | Flaked soft wheat mix 1 | |
| Einkorn (Triticum monococcum) | Flaked einkorn | Precooked einkorn |
| Emmer (Triticum dicoccum) | Flaked emmer | |
| Barley | Flaked barley | |
| Oat | Flaked oat | |
| Millet | Flaked millet | Precooked millet |
| Legumes | ||
| Peas | Flaked peas | Precooked peas |
| Chickpeas | Flaked chickpeas | Precooked chickpeas |
| Flour/Processing | Starch (g/100 g) | Crude Proteins (g/100 g) |
|---|---|---|
| Cereals | ||
| Soft wheat mix/raw | 70 ± 14 | 10 ± 2 |
| Soft wheat mix/flaked | 68 ± 14 | 8 ± 2 |
| Einkorn/raw | 63 ± 13 | 12 ± 2 |
| Einkorn/flaked | 61 ± 12 | 9 ± 2 |
| Einkorn/precooked | 61 ± 12 | 12 ± 2 |
| Emmer/raw | 63 ± 13 | 12 ± 3 |
| Emmer/flaked | 66 ±13 | 12 ± 2 |
| Barley/raw | 69 ± 14 | 8 ± 2 |
| Barley/flaked | 66 ± 13 | 9 ± 2 |
| Barley/precooked | 70 ± 14 | 9 ± 2 |
| Oat/raw | 68 ± 14 | 8 ± 2 |
| Oat/flaked | 62 ± 12 | 13 ± 2 |
| Millet/raw | 68 ± 14 | 12 ± 3 |
| Millet/flaked | 72 ± 14 | 12 ± 2 |
| Millet/precooked | 68 ± 14 | 11 ± 3 |
| Legumes | ||
| Peas/faw | 51 ± 10 | 21 ± 3 |
| Peas/flaked | 54 ± 11 | 19 ± 2 |
| Peas/precooked | 59 ± 12 | 16 ± 2 |
| Chickpeas/raw | 50 ± 9 | 23 ± 3 |
| Chickpeas/flaked | 45 ± 9 | 21 ± 3 |
| Chickpeas/precooked | 48 ± 10 | 21 ± 3 |
| Flour/Processing | Total Polyphenolic Content (TPC, mg GAE/100 g) | Total Flavonoid Content (TFC, mg CE/100 g) | ||
|---|---|---|---|---|
| Free | Bound | Free | Bound | |
| Cereals | ||||
| Soft wheat mix/raw | 65.5 ± 1.1 (45%) aA | 78.7 ± 0.3 (55%) aA | 36.2 ± 2.4 (51%) aA | 35.2 ± 0.2 (49%) aA |
| Soft wheat mix/flaked | 51.3 ± 2.1 (28%) B | 130.5 ± 0.8 (72%) B | 30.0 ± 1.4 (38%) B | 50.0 ± 1.9 (62%) B |
| Einkorn/raw | 54.0 ± 1.3 (48%) bA | 58.4 ± 0.1 (52%) bA | 29.4 ± 0.4 (50%) bA | 29.9 ± 0.1 (50%) bA |
| Einkorn/flaked | 38.5 ± 3.2 (31%) B | 85.5 ± 0.6 (69%) B | 30.0 ± 0.7 (57%) A | 22.7 ± 1.7 (43%) B |
| Einkorn/precooked | 14.0 ± 1.2 (45%) C | 17.3 ± 0.8 (55%) C | 10.1 ± 0.2 (41%) B | 14.4 ± 1.7 (59%) C |
| Emmer/raw | 29.9 ± 1.0 (24%) cA | 93.7 ± 2.0 (76%) cA | 30.5 ± 3.9 (44%) bA | 38.5 ± 0.5 (56%) cA |
| Emmer/flaked | 42.9 ± 0.7 (35%) B | 81.2 ± 1.8 (65%) B | 24.0 ± 0.7 (43%) B | 31.5 ± 1.3 (57%) B |
| Barley/raw | 65.2 ± 3.7 (52%) aA | 61.2 ± 0.2 (48%) cA | 54.5 ± 1.0 (64%) cA | 30.2 ± 0.8 (36%) bA |
| Barley/flaked | 85.6 ± 2.5 (47%) B | 96.3 ± 3.2 (53%) B | 53.4 ± 1.0 (60%) A | 35.5 ± 1.7 (40%) B |
| Barley/precooked | 57.4 ± 2.4 (75%) C | 19.2 ± 0.4 (25%) C | 34.1 ± 1.7 (91%) B | 3.5 ± 0.1 (9%) C |
| Oat/raw | 58.5 ± 0.8 (41%) dA | 83.1 ± 0.3 (59%) eA | 17.3 ± 0.2 (35%) dA | 32.0 ± 1.0 (65%) dA |
| Oat/flaked | 61.3 ± 0.1 (41%) B | 90.1 ± 2.4 (59%) B | 32.2 ± 0.2 (48%) B | 34.4 ± 0.6 (52%) B |
| Millet/raw | 29.1 ± 0.9 (36%) cA | 51.5 ± 1.2 (64%) fA | 11.2 ± 4.1 (34%) eA | 22.0 ± 1.3 (66%) eA |
| Millet/flaked | 34.0 ± 3.6 (36%) A | 61.0 ± 0.4 (64%) B | 14.8 ± 0.7 (43%) B | 19.9 ± 1.5 (57%) B |
| Millet/precooked | 16.5 ± 0.3 (21%) B | 62.4 ± 1.1 (79%) B | 57.2 ± 2.2 (70%) C | 24.7 ± 0.5 (30%) C |
| Legumes | ||||
| Peas/raw | 30.9 ± 0.9 (74%) cA | 10.6 ± 0.2 (26%) gA | 21.3 ± 0.2 (59%)fA | 14.9 ± 0.1 (41%) fA |
| Peas/flaked | 26.9 ± 3.9 (61%) A | 56.1 ± 2.9 (39%) B | 14.0 ± 0.6 (49%)B | 24.6 ± 0.9 (51%) B |
| Peas/precooked | 32.2 ± 0.5 (60%) A | 21.8 ± 1.1 (40%) C | 20.2 ± 0.6 (62%)A | 12.3 ± 0.1 (38%) C |
| Chickpeas/raw | 42.6 ± 1.0 (76%) eA | 13.5 ± 1.2 (24%) hA | 15.7 ± 1.3 (71%)dA | 6.3 ± 0.5 (29%) gA |
| Chickpeas/flaked | 42.8 ± 4.2 (69%) A | 66.0 ± 1.4 (31%) B | 13.5 ± 0.1 (80%)B | 20.2 ± 1.5 (20%) B |
| Chickpeas/precooked | 34.5 ± 0.2 (69%) B | 15.8 ± 0.3 (31%) C | 14.2 ± 0.8 (65%)AB | 7.6 ± 0.1 (35%) C |
| Flour/Processing | TEAC/ABTS (μmol TE/100 g) | TEAC/DPPH (μmol TE/100 g) | ||
|---|---|---|---|---|
| Free | Bound | Free | Bound | |
| Cereals | ||||
| Soft wheat mix/raw | 337.0 ± 7.1 (31%) aA | 754.3 ± 1.6 (69%) aA | 157.8 ± 1.3 (43%) aA | 205.3 ± 11.1 (57%) aA |
| Soft wheat mix/flaked | 418.2 ± 16.1 (24%) B | 1324.0 ± 24.6 (76%) B | 197.1 ± 9.7 (40%) B | 299.8 ± 0.4 (60%) B |
| Einkorn/raw | 271.8 ± 3.0 (29%) bA | 651.1 ± 25.3 (71%) bA | 113.3 ± 2.6 (40%) bA | 172.1 ± 2.5 (60%) bA |
| Einkorn/flaked | 160.4 ± 0.4 (27%) B | 439.0 ± 1.2 (73%) B | 154.0 ± 0.4 (48%) B | 165.2 ± 0.5 (52%) B |
| Einkorn/precooked | 118.3 ± 3.2 (65%) C | 64.2 ± 0.3 (35%) C | 38.6 ± 1.0 (75%) C | 12.8 ± 0.1 (25%) C |
| Emmer/raw | 55.9 ± 3.5 (6%) cA | 949.8 ± 33.0 (94%) cA | 51.5 ± 3.9 (16%) cA | 265.3 ± 0.7 (84%) cA |
| Emmer/flaked | 241.8 ± 1.9 (21%) B | 907.5 ± 3.9 (79%) B | 90.8 ± 0.7 (31%) B | 202.2 ± 1.6 (69%) B |
| Barley/raw | 373.5 ± 3.3 (36%) dA | 678.1 ± 5.0 (64%) dA | 234.7 ± 10.4 (55%) dA | 195.8 ± 1.3 (45%) dA |
| Barley/flaked | 502.6 ± 11.8 (33%) B | 998.8 ± 74.8 (67%) B | 426.8 ± 10.0 (65%) B | 230.5 ± 5.4 (35%) B |
| Barley/precooked | 269.0 ± 0.8 (76%) C | 86.1 ± 1.9 (24%) C | 224.8 ± 0.7 (96%) C | 9.3 ± 0.2 (4%) C |
| Oat/raw | 271.8 ± 12.2 (28%) bA | 684.6 ± 14.5 (72%) dA | 159.2 ± 5.9 (43%) aA | 215.0 ± 1.9 (57%) eA |
| Oat/flaked | 285.6 ± 2.2 (22%) B | 1035.9 ± 25.7 (78%) B | 119.4 ± 0.9 (37%) B | 203.0 ± 1.6 (63%) B |
| Millet/raw | 180.7 ± 1.6 (23%) eA | 596.6 ± 5.9 (77%) eA | 76.7 ± 0.2 (29%) eA | 192.5 ± 1.0 (71%) dA |
| Millet/flaked | 114.8 ± 0.9 (22%) B | 404.0 ± 3.1 (78%) B | 72.1 ± 0.5 (39%) B | 113.6 ± 0.9 (61%) B |
| Millet/precooked | 75.3 ± 0.8 (13%) C | 523.7 ± 14.0 (87%) C | 40.1 ± 0.4 (18%) C | 181.4 ± 4.8 (82%) C |
| Legumes | ||||
| Peas/raw | 181.1 ± 2.5 (69%) eA | 80.9 ± 6.4 (31%) fA | 88.4 ± 0.3 (65%) fA | 48.1 ± 2.1 (35%) fA |
| Peas/flaked | 163.8 ± 0.7 (72%) B | 64.2 ± 0.3 (28%) B | 99.3 ± 0.4 (89%) B | 12.8 ± 0.1 (11%) B |
| Peas/precooked | 174.4 ± 1.6 (56%) C | 136.4 ± 1.2 (44%) C | 103.7 ± 0.9 (77%) C | 31.4 ± 0.3 (23%) C |
| Chickpeas/raw | 266.3 ± 45.8 (72%) bA | 105.3 ± 4.7 (28%) gA | 121.2 ± 18.1 (63%) gA | 72.6 ± 7.0 (37%) gA |
| Chickpeas/flaked | 158.4 ± 3.6 (65%) B | 86.11.9 (35%) B | 59.4 ± 1.3 (86%) B | 9.3 ± 0.2 (14%) B |
| Chickpeas/precooked | 140.2 ± 0.9 (72%) C | 55.5 ± 0.4 (28%) C | 66.4 ± 0.4 (85%) C | 11.5 ± 0.1 (15%) C |
| TPC | TFC | TEAC/ABTS | |
|---|---|---|---|
| Free fraction | |||
| TFC | 0.378 * | ||
| TEAC/ABTS | 0.861 **** | 0.332 ns | |
| TEAC/DPPH | 0.816 **** | 0.477 * | 0.802 **** |
| Bound fraction | |||
| TFC | 0.884 **** | ||
| TEAC/ABTS | 0.961 **** | 0.902 **** | |
| TEAC/DPPH | 0.951**** | 0.898 **** | 0.956 **** |
| Total (calculated) | |||
| TFC | 0.757 **** | ||
| TEAC/ABTS | 0.956 **** | 0.757 **** | |
| TEAC/DPPH | 0.937 **** | 0.762 **** | 0.908 **** |
| Flour/Processing | Total Carotenoid Content (TCC) | α-TEAC (μmol αTE/100 g) | ||
|---|---|---|---|---|
| β-Carotene Equivalent (mg βCE/100 g) | Lutein Equivalent (mg LE/100 g) | ABTS | DPPH | |
| Cereals | ||||
| Soft wheat mix/raw | 0.330 ± 0.004 aA | 0.238 ± 0.003 aA | 117.7 ± 3.5 aA | 37.1 ± 2.0 aA |
| Soft wheat mix/flaked | 0.437 ± 0.006 B | 0.298 ± 0.004 B | 100.0 ± 11.0 A | 60.8 ± 2.9 B |
| Einkorn/raw | 1.083 ± 0.004 bA | 0.837 ± 0.006 bA | 138.1 ± 6.4 bA | 33.5 ± 1.1 bA |
| Einkorn/flaked | 0.251 ± 0.001 B | 0.159 ± 0.001 B | 99.9 ± 0.9 B | 102.6 ± 0.4 B |
| Einkorn/precooked | 2.601 ± 0.008 C | 1.264 ± 0.001 C | 105.0 ± 4.7 C | 27.1 ± 0.2 C |
| Emmer/raw | 0.373 ± 0.013 cA | 0.253 ± 0.013 cA | 163.4 ± 5.4 cA | 39.1 ± 0.8 cA |
| Emmer/flaked | 0.331 ± 0.027 B | 0.197 ± 0.021 B | 140.2 ± 2.6 B | 47.1 ± 0.1 B |
| Barley/raw | 0.407 ± 0.004 dA | 0.284 ± 0.003 dA | 224.8 ± 2.6 dA | 98.8 ± 3.6 dA |
| Barley/flaked | 0.336 ± 0.026 B | 0.216 ± 0.017 B | 218.1 ± 2.6 B | 135.8 ± 1.6 B |
| Barley/precooked | 0.603 ± 0.018 C | 0.340 ± 0.011 C | 216.0 ± 1.0 B | 103.3 ± 0.5 C |
| Oat/raw | 0.463 ± 0.016 eA | 0.283 ± 0.010 dA | 154.7 ± 0.1 eA | 82.6 ± 1.3 eA |
| Oat/flaked | 0.211 ± 0.006 B | 0.145 ± 0.004 B | 138.4 ± 4.3 B | 42.3 ± 0.5 B |
| Millet/raw | 0.759 ± 0.007 fA | 0.562 ± 0.005 eA | 55.2 ± 1.9 fA | 21.4 ± 1.4 fA |
| Millet/flaked | 0.104 ± 0.008 B | 0.057 ± 0.005 B | 50.3 ± 6.0 A | 30.1 ± 0.4 B |
| Millet/precooked | 0.334 ± 0.025 C | 0.221 ± 0.024 C | 39.4 ± 2.4 C | 20.5 ± 0.3 A |
| Legumes | ||||
| Peas/raw | 2.193 ± 0.010 gA | 2.286 ± 0.002 fA | 68.2 ± 0.5 gA | 27.6 ± 1.2 gA |
| Peas/flaked | 0.683 ± 0.011 B | 0.493 ± 0.008 B | 41.4 ± 3.1 B | 22.4 ± 0.1 B |
| Peas/precooked | 2.771 ± 0.022 C | 1.471 ± 0.006 C | 57.4 ± 4.5 C | 29.1 ± 0.4 C |
| Chickpeas/raw | 1.342 ± 0.008 hA | 0.934 ± 0.002 gA | 60.9 ± 0.6 hA | 34.3 ± 0.1 bA |
| Chickpeas/flaked | 0.282 ± 0.012 B | 0.167 ± 0.008 B | 26.2 ± 0.6 B | 26.1 ± 0.3 B |
| Chickpeas/precooked | 1.453 ± 0.016 C | 1.036 ± 0.016 C | 43.8 ± 0.1 C | 44.0 ± 0.2 C |
| Flour/Processing | Chlorophyll a (mg/100 g) | Chlorophyll b (mg/100 g) | Chlorophyll a + b (mg/100 g) | Chlorophyll a/b |
|---|---|---|---|---|
| Chlorophylls (actually present) | ||||
| Peas/raw | 2.92 ± 0.10 a | 1.79 ± 0.01 a | 4.72 ± 0.11 a | 1.6 |
| Peas/flaked | 0.21 ± 0.03 b | 0.041 ± 0.001 b | 0.25 ± 0.03 b | 5.1 |
| Peas/precooked | 0.24 ± 0.01 b | 0.21 ± 0.01 c | 0.45 ± 0.01 c | 1.1 |
| Estimation of chlorophylls before pretreatments (chlorophylls + pheophytins) | ||||
| Peas/raw | 3.96 ± 0.07 c | 2.60 ± 0.02 d | 6.55 ± 0.08 d | 1.5 |
| Peas/flaked | 3.70 ± 0.07 d | 1.71 ± 0.01 e | 5.41 ± 0.08 e | 2.2 |
| Peas/precooked | 3.64 ± 0.01 d | 1.86 ± 0.01 f | 5.50 ± 0.02 e | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consumi, M.; Tamasi, G.; Bonechi, C.; Andreassi, M.; Leone, G.; Magnani, A.; Rossi, C. Effect of Flaking and Precooking Procedures on Antioxidant Potential of Selected Ancient Cereal and Legume Flours. Foods 2022, 11, 1592. https://doi.org/10.3390/foods11111592
Consumi M, Tamasi G, Bonechi C, Andreassi M, Leone G, Magnani A, Rossi C. Effect of Flaking and Precooking Procedures on Antioxidant Potential of Selected Ancient Cereal and Legume Flours. Foods. 2022; 11(11):1592. https://doi.org/10.3390/foods11111592
Chicago/Turabian StyleConsumi, Marco, Gabriella Tamasi, Claudia Bonechi, Marco Andreassi, Gemma Leone, Agnese Magnani, and Claudio Rossi. 2022. "Effect of Flaking and Precooking Procedures on Antioxidant Potential of Selected Ancient Cereal and Legume Flours" Foods 11, no. 11: 1592. https://doi.org/10.3390/foods11111592