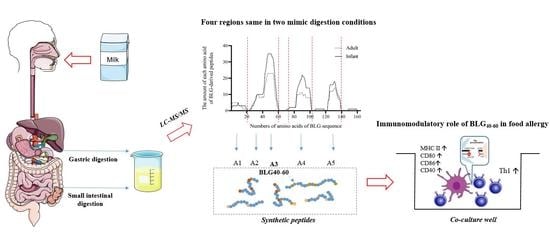
Immunomodulatory Role of BLG-Derived Peptides Based on Simulated Gastrointestinal Digestion and DC-T Cell from Mice Allergic to Cow’s Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulated Gastric and Intestinal Digestion of Bovine Milk
2.2. Protein Profile after Simulated Gastric and Intestinal Digestion
2.3. Synthetic Peptides and Reagents
2.4. Establishment of Caco-2 Monolayers and the Absorption Rate of Digested Peptides
2.5. Animals and Immunization Protocol
2.6. Separation of Cells in the LP
2.7. Separation of Cells in Mesenteric Lymph Nodes
2.8. CD4+ T Cell Sorting and CFSE Staining
2.9. Cell Stimulation and Cytokine Measurements
2.10. Flow Cytometry and FACS Analysis
2.11. Statistical Analysis
3. Results
3.1. Protein Profile of Simulated Digestion In Vitro
3.2. The Absorption Rate of BLG-Derived Peptides
3.3. Effects of BLG-Derived Peptides on LPDC Maturation
3.4. The Effects of BLG-Derived Peptides on the Proliferation and Polarization of CD4+ T Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLG | β-Lactoglobulin |
| LP | Lamina propria cells |
| CMPA | Cow’s milk protein allergy |
| ALA | α-Lactalbumin |
| BCGs | BLG–casein glycomacropeptide mixtures |
| MLN | Mesenteric lymph nodes |
| DC(s) | Dendritic cell(s) |
| LPDCs | LP-derived DC cells |
| OVA | Ovalbumin |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| TEER | Transepithelial electrical resistance |
| Papp | Apparent permeability coefficient |
| Ig | Immunoglobulin |
| DTT | Dithiothreitol |
| EDTA | Ethylene diamine tetraacetie acid |
| D-HBSS | Hank’s balanced salt solution without calcium and magnesium |
| RPMI-1640 | Roswell Park Memorial Institute 1640 |
| Con-A | Concanavalin A |
| MWs | Molecular weights |
| CN | Casein |
References
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Tan, M.; Wu, J.; Chen, Z.; Long, X.; Zeng, Y.; Cai, H.; Zhang, Y.; Geng, L.; Xiao, Y.; et al. Prevalence, Characteristics, and Outcome of Cow’s Milk Protein Allergy in Chinese Infants: A Population-Based Survey. JPEN J. Parenter Enter. Nutr. 2019, 43, 803–808. [Google Scholar] [CrossRef]
- Hattori, M.; Miyakawa, S.; Ohama, Y.; Hattori, M.; Kawamura, H.; Yoshida, K.; To-o, T.; Kuriki, T.; Takahashi, K. Reduced Immunogenicity of beta-Lactoglobulin by Conjugation with Acidic Oligosaccharides. J. Agric. Food Chem. 2004, 52, 4546–4553. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Ferranti, P.; Fierro, O.; Mamone, G.; Caira, S.; Di Luccia, A.; Monica, S.; Addeo, F. Peptides surviving the simulated gastrointestinal digestion of milk proteins: Biological and toxicological implications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Iacomino, G.; Mamone, G.; Ferranti, P.; Fierro, O.; Gianfrani, C.; Di Luccia, A.; Addeo, F. Transport across Caco-2 monolayers of peptides arising from in vitro digestion of bovine milk proteins. Food Chem. 2013, 139, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.J.; Martos, G.; Molina, E.; Pilosof, A.M. Reduced beta-lactoglobulin IgE binding upon in vitro digestion as a result of the interaction of the protein with casein glycomacropeptide. Food Chem. 2016, 192, 943–949. [Google Scholar] [CrossRef]
- Meulenbroek, L.A.; van Esch, B.C.; Hofman, G.A.; den Hartog Jager, C.F.; Nauta, A.J.; Willemsen, L.E.; Bruijnzeel-Koomen, C.A.; Garssen, J.; van Hoffen, E.; Knippels, L.M. Oral treatment with beta-lactoglobulin peptides prevents clinical symptoms in a mouse model for cow’s milk allergy. Pediatric Allergy Immunol. Off. Publ. Eur. Soc. Pediatric Allergy Immunol. 2013, 24, 656–664. [Google Scholar] [CrossRef]
- Kostadinova, A.I.; Meulenbroek, L.A.; van Esch, B.C.; Hofman, G.A.; Garssen, J.; Willemsen, L.E.; Knippels, L.M. A Specific Mixture of Fructo-Oligosaccharides and Bifidobacterium breve M-16V Facilitates Partial Non-Responsiveness to Whey Protein in Mice Orally Exposed to beta-Lactoglobulin-Derived Peptides. Front. Immunol. 2016, 7, 673. [Google Scholar] [CrossRef] [Green Version]
- Gouw, J.W.; Jo, J.; Meulenbroek, L.; Heijjer, T.S.; Kremer, E.; Sandalova, E.; Knulst, A.C.; Jeurink, P.V.; Garssen, J.; Rijnierse, A.; et al. Identification of peptides with tolerogenic potential in a hydrolysed whey-based infant formula. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 1345–1353. [Google Scholar] [CrossRef] [Green Version]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [CrossRef]
- Zhao, D.; Le, T.T.; Nielsen, S.D.; Larsen, L.B. Effect of Storage on Lactase-Treated beta-Casein and beta-Lactoglobulin with Respect to Bitter Peptide Formation and Subsequent in Vitro Digestibility. J. Agric. Food Chem. 2017, 65, 8409–8417. [Google Scholar] [CrossRef] [PubMed]
- Macierzanka, A.; Sancho, A.I.; Mills, E.N.C.; Rigby, N.M.; Mackie, A.R. Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin. Soft Matter 2009, 5, 538–550. [Google Scholar] [CrossRef]
- Bossios, A.; Theodoropoulou, M.; Mondoulet, L.; Rigby, N.M.; Papadopoulos, N.G.; Bernard, H.; Adel-Patient, K.; Wal, J.-M.; EN Mills, C.; Papageorgiou, P. Effect of simulated gastro-duodenal digestion on the allergenic reactivity of beta-lactoglobulin. Clin. Transl. Allergy 2011, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Benedé, S.; López-Expósito, I.; Giménez, G.; Grishina, G.; Bardina, L.; Sampson, H.A.; López-Fandiño, R.; Molina, E. Mapping of IgE epitopes in in vitro gastroduodenal digests of β-lactoglobulin produced with human and simulated fluids. Food Res. Int. 2014, 62, 1127–1133. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Nutten, S.; Bernard, H.; Fritsche, R.; Ah-Leung, S.; Meziti, N.; Prioult, G.; Mercenier, A.; Wal, J.M. Immunomodulatory potential of partially hydrolyzed beta-lactoglobulin and large synthetic peptides. J. Agric. Food Chem. 2012, 60, 10858–10866. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Matsushita, S.; Kaneko, H.; Shinoda, S.; Sakaguchi, H.; Nishimura, Y.; Kondo, N. Identification of Beta-Lactoglobulin-Derived Peptides and Class II HLA Molecules Recognized by T Cells From Patients With Milk Allergy. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2001, 31, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Do, A.B.; Williams, K.; Toomer, O.T. In vitro digestibility and immunoreactivity of bovine milk proteins. Food Chem. 2016, 190, 581–587. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Dupont, D.; Mandalari, G.; Molle, D.; Jardin, J.; Leonil, J.; Faulks, R.M.; Wickham, M.S.; Mills, E.N.; Mackie, A.R. Comparative resistance of food proteins to adult and infant in vitro digestion models. Mol. Nutr. Food Res. 2010, 54, 767–780. [Google Scholar] [CrossRef]
- Susewind, J.; de Souza Carvalho-Wodarz, C.; Repnik, U.; Collnot, E.M.; Schneider-Daum, N.; Griffiths, G.W.; Lehr, C.M. A 3D co-culture of three human cell lines to model the inflamed intestinal mucosa for safety testing of nanomaterials. Nanotoxicology 2016, 10, 53–62. [Google Scholar] [CrossRef]
- Li, X.M.; Schofield, B.H.; Huang, C.K.; Kleiner, G.I.; Sampson, H.A. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999, 103, 206–214. [Google Scholar] [CrossRef]
- Weigmann, B.; Tubbe, I.; Seidel, D.; Nicolaev, A.; Becker, C.; Neurath, M.F. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat. Protoc. 2007, 2, 2307–2311. [Google Scholar] [CrossRef] [PubMed]
- Denning, T.L.; Norris, B.A.; Medina-Contreras, O.; Manicassamy, S.; Geem, D.; Madan, R.; Karp, C.L.; Pulendran, B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J. Immunol. 2011, 187, 733–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamourthy, G.; Arias, M.; Nguyen, L.T.; Ishida, H.; Vogel, H.J. Expression and Purification of Chemokine MIP-3α (CCL20) through a Calmodulin-Fusion Protein System. Microorganisms 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Bouzerzour, K.; Morgan, F.; Cuinet, I.; Bonhomme, C.; Jardin, J.; Huerouluron, I.L.; Dupont, D. In vivo digestion of infant formula in piglets: Protein digestion kinetics and release of bioactive peptides. Br. J. Nutr. 2012, 108, 2105–2114. [Google Scholar] [CrossRef] [Green Version]
- Mamoru Totsuka; Akio Ametani; Kaminogawa, S. Fine mapping of T-cell determinants of bovine β-lactoglobin. Cytotechnology 1997, 25, 101–113. [Google Scholar] [CrossRef]
- Nordgren, A.; Karlsson, T.F.; Wiklund, L. Glutamine concentration and tissue exchange with intravenously administered alpha-ketoglutaric acid and ammonium: A dose-response study in the pig. Nutrition 2002, 18, 496–504. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Spera, I.; Menga, A.; Infantino, V.; Iacobazzi, V.; Castegna, A. Glutamine synthetase desensitizes differentiated adipocytes to proinflammatory stimuli by raising intracellular glutamine levels. FEBS Lett. 2014, 588, 4807–4814. [Google Scholar] [CrossRef] [Green Version]
- Fillmann, H.; Kretzmann, N.A.; San-Miguel, B.; Llesuy, S.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology 2007, 236, 217–226. [Google Scholar] [CrossRef]
- Chellamuthu, V.R.; Ermilova, E.; Lapina, T.; Lüddecke, J.; Minaeva, E.; Herrmann, C.; Hartmann, M.D.; Forchhammer, K. A widespread glutamine-sensing mechanism in the plant kingdom. Cell 2014, 159, 1188–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Li, H.; Huang, N.; Tian, J.; Liu, Z.; Zhou, X.; Yao, K.; Li, T.; Yin, Y. Effects of Alpha-Ketoglutarate on Glutamine Metabolism in Piglet Enterocytes in Vivo and in Vitro. J. Agric. Food Chem. 2016, 64, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hou, H.; Wang, S.; Zhao, X.; Li, B. Effects of early enteral nutrition supplemented with collagen peptides on post-burn inflammatory responses in a mouse model. Food Funct. 2017, 8, 1933–1941. [Google Scholar] [CrossRef]
- Japelj, N.; Suligoj, T.; Zhang, W.; Côrte-Real, B.; Messing, J.; Ciclitira, P.A.-O. Natural variants of α-gliadin peptides within wheat proteins with reduced toxicity in coeliac disease. Br. J. Nutr. 2020, 124, 480. [Google Scholar] [CrossRef]
- Louis-Auguste, J.; Besa, E.; Zyambo, K.; Munkombwe, D.; Banda, R.; Banda, T.; Watson, A.; Mayneris-Perxachs, J.; Swann, J.; Kelly, P. Tryptophan, glutamine, leucine, and micronutrient supplementation improves environmental enteropathy in Zambian adults: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1240–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alashkar Alhamwe, B.; Meulenbroek, L.; Veening-Griffioen, D.H.; Wehkamp, T.M.D.; Alhamdan, F.; Miethe, S.; Harb, H.; Hogenkamp, A.; Knippels, L.M.J.; Pogge von Strandmann, E.; et al. Decreased Histone Acetylation Levels at Th1 and Regulatory Loci after Induction of Food Allergy. Nutrients 2020, 12, 3193. [Google Scholar] [CrossRef]
- Abbring, S.; Wolf, J.; Ayechu-Muruzabal, V.; Diks, M.A.P.; Alhamwe, B.A.; Alhamdan, F.; Harb, H.; Renz, H.; Garn, H.; Garssen, J.; et al. Raw Cow’s Milk Reduces Allergic Symptoms in a Murine Model for Food Allergy-A Potential Role For Epigenetic Modifications. Nutrients 2019, 11, 1721. [Google Scholar] [CrossRef] [Green Version]
- Kroghsbo, S.; Christensen, H.R.; Frokiaer, H. Experimental parameters differentially affect the humoral response of the cholera-toxin-based murine model of food allergy. Int. Arch. Allergy Immunol. 2003, 131, 256–263. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Bernard, H.; Ah-Leung, S.; Creminon, C.; Wal, J.M. Peanut- and cow’s milk-specific IgE, Th2 cells and local anaphylactic reaction are induced in Balb/c mice orally sensitized with cholera toxin. Allergy 2005, 60, 658–664. [Google Scholar] [CrossRef]





| Protein | Unique Peptides | Sequence Coverage (%) | Mass Range (Da) | Score Range (>20) |
|---|---|---|---|---|
| BLG | 109 | 72.5 | 813.42–2218.22 | 20.12–172.74 |
| ALA | 23 | 43.7 | 843.41–1596.69 | 22.01–106.91 |
| αs1-CN | 210 | 79.4 | 842.45–2687.35 | 20.14–245.55 |
| αs2-CN | 79 | 61.7 | 811.44–1768.95 | 20.97–203.70 |
| β-CN | 217 | 88.4 | 837.44–2845.34 | 20.03–176.25 |
| κ-CN | 58 | 62.6 | 776.39–2138.14 | 20.70–106.42 |
| Unique Peptides in Infant Digestive Condition | Unique Peptides in Adult Digestive Condition |
|---|---|
| TPEVDDEALEKF | SDISLLDAQSAPLRV |
| TPEVDDEALEKFD | KPTPEGDL |
| VEELKPTPEGD | ALEKFDKA |
| KIDALNENKVLVLDTDYKK | SDISLLDAQ |
| PEVDDEALEKFDK | FKIDALNE |
| VYVEELKPTPEGDLE | ELKPTPEGDL |
| LNENKVLV | LIVTQTMKGLDIQK |
| VYVEELKPTPEGDL | LIVTQTMKGLDIQ |
| VEELKPTPEGDLEILLQK | |
| VYVEELKPTPEGDLEIL | |
| KPTPEGDLE | |
| FKIDALNENKV | |
| NKVLVLDTDYK | |
| VLVLDTDYKKY | |
| IDALNENKVLVLDTDYK | |
| ELKPTPEGDLEI | |
| DALNENKV | |
| KPTPEGDLEIL | |
| GLDIQKVAGT | |
| NENKVLVLDTDY | |
| YVEELKPTPE | |
| ASDISLLDAQSAPL | |
| VEELKPTPE | |
| FKIDALNENKVL | |
| SLLDAQSAPLR | |
| GLDIQKVAGTWY | |
| LKPTPEGDLEI | |
| MAASDISLLDAQSAPL | |
| RVYVEELKPTPEGD | |
| ALNENKVL | |
| YVEELKPTPEGDLEILLQ | |
| LKPTPEGDLEILLQ | |
| MENSAEPEQSLA | |
| RTPEVDDEA | |
| DALNENKVLV |
| Peptides | Amino Acid Sequences | Purity |
|---|---|---|
| A1(BLG-AA1–14) | LIVTQTMKGLDIQK | >90% |
| A2(BLG-AA24–35) | MAASDISLLDAQ | >90% |
| A3( BLG-AA40–60) | RVYVEELKPTPEGDLEILLQK | >90% |
| A4(BLG-AA82–101) | FKIDALNENKVLVLDTDYKK | >90% |
| A5(BLG-AA123–139) | VRTPEVDDEALEKFDKA | >90% |
| A6(OVA-AA323–339) | ISQAVHAAHAEINEAGR | >90% |
| Group | Percentage of MLN-T |
|---|---|
| Blank | 74.35 ± 0.21 a |
| A1 | 71.80 ± 0.28 e,f |
| A2 | 71.65 ± 0.21 f |
| A3 | 70.90 ± 0.14 g |
| A4 | 72.80 ± 0.14 b |
| A5 | 72.1 ± 0.00 c,d,e |
| A6 | 71.95 ± 0.07 d,e,f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Yang, F.; Meng, X.; Wu, Y.; Tong, P.; Gao, J.; Chen, H.; Li, X. Immunomodulatory Role of BLG-Derived Peptides Based on Simulated Gastrointestinal Digestion and DC-T Cell from Mice Allergic to Cow’s Milk. Foods 2022, 11, 1450. https://doi.org/10.3390/foods11101450
Ma X, Yang F, Meng X, Wu Y, Tong P, Gao J, Chen H, Li X. Immunomodulatory Role of BLG-Derived Peptides Based on Simulated Gastrointestinal Digestion and DC-T Cell from Mice Allergic to Cow’s Milk. Foods. 2022; 11(10):1450. https://doi.org/10.3390/foods11101450
Chicago/Turabian StyleMa, Xin, Fan Yang, Xuanyi Meng, Yong Wu, Ping Tong, Jinyan Gao, Hongbing Chen, and Xin Li. 2022. "Immunomodulatory Role of BLG-Derived Peptides Based on Simulated Gastrointestinal Digestion and DC-T Cell from Mice Allergic to Cow’s Milk" Foods 11, no. 10: 1450. https://doi.org/10.3390/foods11101450