Insights into the Composition and Antibacterial Activity of Amomum tsao-ko Essential Oils from Different Regions Based on GC-MS and GC-IMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. Extraction of AEOs
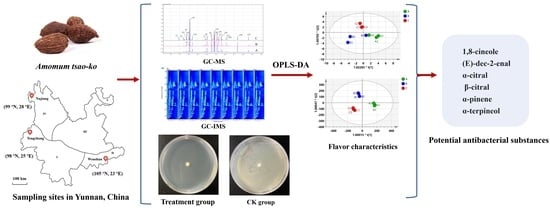
2.3. GC-MS Analysis
2.4. GC-IMS Analysis
2.5. Agar Disc Diffusion Assay
2.6. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of AEOs by GC-MS
3.2. Composition of AEOs by GC-IMS
3.3. Analysis of Compounds Detected in AEOs from Different Regions
3.4. Multivariate Analysis of Compounds of AEOs from Different Regions Based on PCA and OPLS-DA
3.5. Antibacterial Activity of the AEOs from Different Regions
3.5.1. Antibacterial Activity of the AEOs
3.5.2. Main Antibacterial Substances Analysis of AEOs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A. tsao-ko | Amomun tsao-ko |
| EOs | Essential oils |
| AEO | A. tsao-ko essential oil |
| AEOs | A. tsao-ko essential oils |
| S. aureus | Staphylococcus aureus |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-IMS | Gas chromatography–ion mobility spectrometry |
| VOCs | Volatile organic compounds |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| PCA | Principal component analysis |
| Dt | Drift time |
| Rt | Retention time |
| RI | Retention index |
| SD | Standard deviation |
| VIP | Variable importance of projection |
| OPLS-DA | Orthogonal partial least square discriminant analysis |
| DDs | Disc diameters of inhibition zones |
| MA | Multivariate analysis |
References
- Xu, Z.H.; Yang, S.B.; Yang, T.M.; Yang, W.Z.; Wang, Y.Z.; Zhang, J.Y. Analysis on geographical distribution and phenotypic variation of Amomum tsaoko Crevost & lemarié. J. Plant Genet. Resour. 2021, 22, 1009–1020. (In Chinese) [Google Scholar]
- Qin, H.W.; Wang, Y.Z.; Yang, M.Q.; Yang, S.B.; Zhang, J.Y. Medicinal textual research of Tsaokofructus. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 139–148. (In Chinese) [Google Scholar]
- Zhang, W.; Yang, S.C.; Wei, X.; Xie, S.Q. Developing status and strategies of Amomum tsao-ko plantation in Yunnan. Chin. Med. Mater.-World Sci. Technol. 2011, 13, 899–903. (In Chinese) [Google Scholar]
- Liu, H.; Yan, Q.S.; Zou, D.L.; Bu, X.L.; Zhang, B.J.; Ma, X.C.; Leng, A.L.; Zhang, H.L.; Li, D.W.; Wang, C. Identification and bioactivity evaluation of ingredients from the fruits of Amomum tsaoko Crevost et Lemaire. Phytochem. Lett. 2018, 28, 111–115. [Google Scholar] [CrossRef]
- Seong, S.H.; Ji, H.L.; Yun-Hyeok, H.C.; Wonsik, J.; Eun-KyungAhn, K.A.; Seung, H.L.; Joa, S.O. Amotsaokonal A–C, benzaldehyde and cycloterpenal from Amomum tsao-ko. Tetrahedron Lett. 2015, 56, 6681–6684. [Google Scholar]
- Falleh, H.; Ben, J.M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 8. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.J.; Gao, K. The research on the preservation effect of peach fruit by clove essential oil microcapsule. Packag. Food Mach. 2015, 33, 19–23. (In Chinese) [Google Scholar]
- Xing, Y.; Li, X.H.; Xu, Q.L.; Yun, J.; Lu, Y.Q.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Jemaa, M.B.; Falleh, H.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Quality preservation of deliberately contaminated milk using thyme free and nanoemulsified essential oils. Food Chem. 2017, 217, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yan, R.W.; Cai, X.Q.; Zheng, Z.L.; Zou, G.L. Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J. Sci. Food Agric. 2008, 88, 2111–2116. [Google Scholar] [CrossRef]
- Sun, W.M.; Ma, Y.N.; Yin, Y.J.; Chen, C.J.; Xu, F.R.; Dong, X.; Cheng, Y.X. Effects of essential oils from Zingiberaceae plants on root-rot disease of panax notoginseng. Molecules 2018, 23, 1021. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Wang, L.T.; Liu, J.Z.; Wang, H.M.; Guo, N.; Gu, C.B.; Fu, Y.J. Rapid extraction of Amomum tsao-ko essential oil and determination of its chemical composition, antioxidant and antimicrobial activities. J. Chromatogr. B 2017, 1061–1062, 364–371. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Hu, J.; Ling, R.M.; Li, P.L.; Yan, H.B.; Liu, Y.Y.; Yang, Y.; Yang, Q.; Wu, L.Z.; Zhang, Z.K.; Yan, J. Effect on volatile components of Amomum tsao-ko by four different preprocessing models. Chin. J. Trop. Crop. 2019, 40, 773–780. (In Chinese) [Google Scholar]
- Wang, J.J.; Li, Y.K.; Lu, Q.W.; Hu, Q.Y.; Liu, P.H.; Yang, Y.W.; Li, G.D.; Xie, H.; Tang, H.R. Drying temperature affects essential oil yield and composition of black cardamom (Amomum tsao-ko). Ind. Crops Prod. 2021, 168, 113580. [Google Scholar] [CrossRef]
- Gu, F.L.; Zhang, L.H.; Fang, Y.M.; Liu, G.H.; Shen, S.B.; Jiang, T.L.; Duan, C.F.; Song, J.M.; Cai, Y.Y.; Huang, F.F. Analysis of physical properties, essential oil content and composition of Amomum tsao-ko from different origins of Yunnan. Chin. J. Trop. Crop. 2018, 39, 1440–1446. (In Chinese) [Google Scholar]
- Li, G.D.; Lu, Q.W.; Wang, J.J.; Hu, Q.Y.; Liu, P.H.; Yang, Y.W.; Li, W.K.; Tang, H.R.; Xie, H. Correlation analysis of compounds in essential oil of Amomum tsaoko seed and fruit morphological characteristics, geographical conditions, locality of growth. Agronomy 2021, 11, 744. [Google Scholar] [CrossRef]
- Liu, N.; Xia, X.S.; Zhao, Y.; Su, Y.; Sun, J.R. GC-MS analysis and antibacterial test of essential oil of Amomum tsao-ko from different regions. J. Yunnan Natl. Univ. 2021, 30, 97–103. (In Chinese) [Google Scholar]
- Li, W.Q.; Chen, Y.P.; Blank, I.; Li, F.Y.; Li, C.B.; Liu, Y. GC x GC-ToF-MS and GC-IMS based volatile profile characterization of the Chinese dry-cured hams from different regions. Food Res. Int. 2021, 142, 110222. [Google Scholar] [CrossRef]
- Tang, N.; Liu, J.Y.; Chen, X.A.; Yang, Y.J.; Chen, S.X.; Zhou, A.M. Analysis of volatile components in essential oil of finger citron from Guangdong province at different picking times by GC-MS and GC-IMS. Food Sci. 2021, 42, 193–202. (In Chinese) [Google Scholar]
- Yang, X.S.; Zhu, K.L.; Guo, H.M.; Geng, Y.L.; Lv, W.; Wang, S.Y.; Qin, P.Y.; Gao, Y.Q.; Ren, G.X. Characterization of volatile compounds in differently coloured Chenopodium quinoa seeds before and after cooking by headspace-gas chromatography-ion mobility spectrometry. Food Chem. 2021, 348, 129086. [Google Scholar] [CrossRef]
- Deng, S.Y.; Liu, Y.; Huang, F.; Liu, J.Q.; Han, D.; Zhang, C.H.; Blecker, C. Evaluation of volatile flavor compounds in bacon made by different pig breeds during storage time. Food Chem. 2021, 357, 129765. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhu, Y.C. The floristic division of Yunnan province. In Yunnan Vegetation; Science Press: Beijing, China, 1987; pp. 31–32. [Google Scholar]
- Diao, W.R.; Hu, Q.P.; Feng, S.S.; Li, W.Q.; Xu, J.G. Chemical composition and antibacterial activity of the essential oil from Green Huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J. Agric. Food Chem. 2013, 61, 6044–6049. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; You, C.X.; Wang, C.F.; Yang, K.; Chen, R.; Zhang, W.J.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Chemical constituents and insecticidal activities of the essential oil from Amomum tsaoko against two stored-product insects. J. Oleo Sci. 2014, 63, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.; Tan, S.K.; Kohlenberg, B.; Braun, N.A. Amomum tsao-ko-Chinese black cardamom: Detailed oil composition and comparison with two other cardamom species. Nat. Prod. Commun. 2019, 14, 1934578X19857675. [Google Scholar] [CrossRef] [Green Version]
- Food and Drug Administration Department of Health and Human Services. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Available online: https://www.fda.gov/media/79581/download (accessed on 1 February 2022).
- Wan, P.; Liu, J.; Chen, D.W. Analysis of aroma-active compounds in bighead carp head soup and their influence on umami of a model soup. Microchem. J. 2021, 168, 106436. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: http://flavornet.org/flavornet.html (accessed on 1 February 2021).
- Stojković, D.; Soković, M.; Glamočlija, J.; Džamić, A.; Ćirić, A.; Ristić, M.; Grubišić, D. Chemical composition and antimicrobial activity of Vitex agnus-castus L. fruits and leaves essential oils. Food Chem. 2011, 128, 1017–1022. [Google Scholar] [CrossRef]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yoplac, I.; Vargas, L.; Robert, P.; Hidalgo, A. Characterization and antimicrobial activity of microencapsulated citral with dextrin by spray drying. Heliyon 2021, 7, e06737. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.G.; Chen, F.L.; Guan, H.N.; Liu, L.L.; Zhang, C.Y.; Zhu, P.Y.; Zhang, N. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. alpha-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of hexanal, (E)-2-hexenal, and hexyl acetate to improve the safety of Fresh-Sliced apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Yang, Y.W.; Liu, X.L.; Pu, C.X.; Qian, Z.G.; Guan, K.Y. The influence of altitude and latitude on breeding of Amomum tsaoko (Zingiberaceae). J. Biosci. Med. 2014, 2, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Susanne, W.; Erik, J.; Lina, S.; Ewa, J.M.; Ulf, E.; John, P.S.; Johan, G.; Thomas, M.; Johan, T. Visualization of GC/TOF-MS-Based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar]
- Huang, D.F.; Xu, J.G.; Liu, J.X.; Zhang, H.; Hu, Q.P. Chemical constituents, antibacterial activity and mechanism of action of the essential oil from Cinnamomum cassia bark against four food-related bacteria. Microbiology 2014, 83, 357–365. [Google Scholar] [CrossRef]
- Kuhn, D.; Ziem, R.; Scheibel, T.; Buhl, B.; Vettorello, G.; Pacheco, L.A.; Daiane, H.D.; Kauffmann, C.; Freitasa, E.M.; Ethura, E.M.; et al. Antibiofilm activity of the essential oil of Campomanesia aurea O. Berg against microorganisms causing food borne diseases. LWT 2019, 108, 247–252. [Google Scholar] [CrossRef]
- Samadi, N.; Manayi, A.; Vazirian, M.; Samadi, M.; Zeinalzadeh, Z.; Saghari, Z.; Abadian, N.; Mozaffarian, V.O.A.; Khanaviet, M. Chemical composition and antimicrobial activity of the essential oil of Anthemis altissima L. var. altissima. Nat. Prod. Res. 2012, 26, 1931–1934. [Google Scholar] [CrossRef]
- Braham, M.; Daami-Remadi, M.; Bchir, A.; Jabnoun-Khiareddine, H.; Saidana, D.; Mansour-Gueddes, S.B. Chemical composition and biological activities assess-ment of olive fruit volatiles from different varieties grown in Tunisia. Acta Sci. Pol. Hortorum Cultus 2020, 19, 3–20. [Google Scholar]






| No. | RI 1 | Compound | MF | Concentration(%) * | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| Terpenes(12) | 53.1 | 54.6 | 54.3 | |||
| 1 | 932.5 | -pinene | 0.8 ± 0.2 | 1.0 ± 0.2 | 1.5 ± 0.2 | |
| 2 | 976.6 | (−)--pinene | 0.9 ± | 1.1 ± 0.3 | 1.6 ± 0.3 | |
| 3 | 1005.9 | -phellandrene | 0.4 ± | 2.5 ± | 3.6 ± | |
| 4 | 1024.0 | o-cymene | 1.1 ± 0.1 | 1.9 ± 0.7 | 1.8 ± 0.7 | |
| 5 | 1028.7 | D-limonene | 1.6 ± 0.2 | 2.5 ± 0.8 | 2.1 ± 0.8 | |
| 6 | 1035.4 | 1,8-cineole | 28.9 ± 4.2 | 36.6 ± 6.5 | 29.1 ± 6.5 | |
| 7 | 1179.6 | (−)-4-terpineol | 1.0 ± 0.2 | 0.7 ± 0.5 | 0.6 ± 0.5 | |
| 8 | 1194.2 | -terpineol | 3.0 ± 0.4 | 2.1 ± 1.5 | 3.2 ± 1.5 | |
| 9 | 1237.1 | -citral | 6.5 ± | 3.1 ± | 3.5 ± | |
| 10 | 1267.1 | -citral | 6.9 ± 2.8 | 2.1 ± 1.3 | 3.2 ± 1.3 | |
| 11 | 1369.3 | -methyl-cinnamaldehyde | 0.6 ± 0.6 | 0.5 ± 0.7 | 2.2 ± 0.7 | |
| 12 | 1557.3 | -nerolidol | 1.4 ± | 0.5 ± | 1.9 ± | |
| Aldehydes(7) | 30.2 | 25.8 | 25.2 | |||
| 13 | 1002.7 | octanal | 0.9 ± | 2.1 ± | 1.5 ± | |
| 14 | 1057.2 | (E)-oct-2-enal | 2.0 ± 0.2 | 2.9 ± 0.8 | 2.1 ± 0.8 | |
| 15 | 1262.2 | (E)-dec-2-enal | 12.0 ± 2.9 | 9.6 ± 3.1 | 8.5 ± 3.1 | |
| 16 | 1291.6 | -methyl-benzenepropanal | 5.5 ± 0.3 | 3.9 ± 1.9 | 5.3 ± 1.9 | |
| 17 | 1328.0 | geranial dimethyl acetal | 3.9 ± 3.0 | 4.8 ± 2.0 | 3.8 ± 2.0 | |
| 18 | 1336.1 | indane-4-carboxaldehyde | 1.4 ± | 0.8 ± | 1.2 ± | |
| 19 | 1463.9 | (E)-dodec-2-enal | 4.5 ± 1.2 | 1.7 ± 1.5 | 2.8 ± 1.5 | |
| Ester(1) | - | - | 2.1 | |||
| 20 | 1373.4 | geranyl acetate | - | - | 2.1 ± 0.0 | |
| No. | Compound | Category | Correlation | p |
|---|---|---|---|---|
| 1 | elemol | Terpene | −0.953 ** | 0.000 |
| 2 | -muurolene | Terpene | −0.949 ** | 0.000 |
| 3 | -nerolidol | Terpene | −0.896 ** | 0.001 |
| 4 | -terpineol(M) | Terpene | −0.896 ** | 0.001 |
| 5 | bornyl acetate | Ester | −0.845 ** | 0.004 |
| 6 | 2-methylbutanal(M) | Aldehyde | −0.843 ** | 0.004 |
| 7 | [(E)-dec-2-enyl] acetate | Ester | −0.804 ** | 0.009 |
| 8 | ethanol(D) | Alcohol | −0.791 * | 0.011 |
| 9 | -muurolene | Terpene | −0.791 * | 0.011 |
| 10 | 4,10-epoxyamorphane | Terpene | −0.791 * | 0.011 |
| 11 | -methyl-cinnamaldehyde | Terpene | −0.750 * | 0.020 |
| 12 | 3-methylbutanal(M) | Aldehyde | −0.738 * | 0.023 |
| 13 | ethanol(T) | Alcohol | −0.738 * | 0.023 |
| 14 | ethyl acetate(M) | Ester | −0.685 * | 0.042 |
| 15 | (E)-dec-2-enal(M) | Aldehyde | −0.685 * | 0.042 |
| 16 | 2-ethylfuran | Other | −0.685 * | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, J.; Qin, Z.; Wang, Y.; Zhao, P.; Gao, H. Insights into the Composition and Antibacterial Activity of Amomum tsao-ko Essential Oils from Different Regions Based on GC-MS and GC-IMS. Foods 2022, 11, 1402. https://doi.org/10.3390/foods11101402
Li W, Li J, Qin Z, Wang Y, Zhao P, Gao H. Insights into the Composition and Antibacterial Activity of Amomum tsao-ko Essential Oils from Different Regions Based on GC-MS and GC-IMS. Foods. 2022; 11(10):1402. https://doi.org/10.3390/foods11101402
Chicago/Turabian StyleLi, Weidan, Junjie Li, Zhen Qin, Yang Wang, Pengyu Zhao, and Haiyan Gao. 2022. "Insights into the Composition and Antibacterial Activity of Amomum tsao-ko Essential Oils from Different Regions Based on GC-MS and GC-IMS" Foods 11, no. 10: 1402. https://doi.org/10.3390/foods11101402