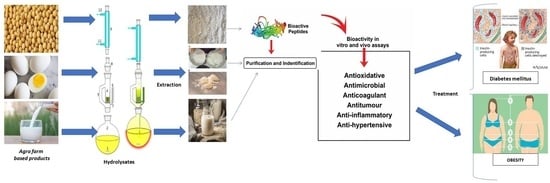
The Role of Bioactive Peptides in Diabetes and Obesity
Abstract
:1. Introduction
1.1. Sources of Bioactive Peptides
1.2. Different Functional Bioactive Peptides
1.2.1. Pharmacological Properties of Bioactive Peptides
1.2.2. Antioxidant Properties of Bioactive Peptides
1.2.3. Antimicrobial Properties of Bioactive Peptides
1.2.4. Immunomodulatory Properties of Bioactive Peptides
1.2.5. Cytomodulatory Properties of Bioactive Peptides
1.2.6. Metabolic Effects of Bioactive Peptides
2. Effects of Bioactive Peptides on Human Health
2.1. Metabolic Effects of Bioactive Peptides
2.2. Cholesterol-Lowering Peptides
2.3. Mechanism of Action of Anti-Diabetic Peptides against Type 2 Diabetes
2.4. Mechanism of Action of Anti-Inflammatory Peptides
3. Diversity in the Production of Bioactive Peptides
3.1. Enzymatic Hydrolysis
3.1.1. In Vitro Study of Egg Hydrolysate (E.H.)/Peptides
3.1.2. In Vitro Study of Egg Hydrolysate
3.1.3. In Vitro Studies of Soy Hydrolysate (S.H.)/Peptides
3.1.4. In Vivo Studies of Soy Hydrolysate (S.H.)/Peptides
3.2. Gastrointestinal Digestion
3.3. Fermentation
3.4. Genetic Engineering
4. Purification and Characterization of Bioactive Peptides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Adje, E.Y.; Balti, R.; Kouach, M.; Dhulster, P.; Guillochon, D.; Nedjar-Arroume, N. Obtaining antimicrobial peptides by controlled peptic hydrolysis of bovine hemoglobin. Int. J. Biol. Macromol. 2011, 49, 143–153. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Adesso, S.; Ostacolo, C.; Sala, M.; Sommella, E.; Scala, M.C.; Messore, A.; Marzocco, S.; Campiglia, P. Antioxidant properties of buffalo-milk dairy products: A β-Lg peptide released after gastrointestinal digestion of buffalo ricotta cheese reduces oxidative stress in intestinal epithelial cells. Int. J. Mol. Sci. 2018, 19, 1955. [Google Scholar] [CrossRef] [Green Version]
- Anadón, A.; Martínez, M.; Ares, I.; Ramos, E.; Martínez-Larrañaga, M.; Contreras, M.; Ramos, M.; Recio, I. Acute and repeated dose (4 weeks) oral toxicity studies of two antihypertensive peptides, RYLGY and AYFYPEL, that correspond to fragments (90–94) and (143–149) from αs1-casein. Food Chem. Toxicol. 2010, 48, 1836–1845. [Google Scholar] [CrossRef]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Spagnoletti Zeuli, P.; Gioia, T.; Logozzo, G. Molecular analysis of the parallel domestication of the common bean (P haseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef]
- Mohan, A.; Rajendran, S.R.; He, Q.S.; Bazinet, L.; Udenigwe, C.C. Encapsulation of food protein hydrolysates and peptides: A review. RSC Adv. 2015, 5, 79270–79278. [Google Scholar] [CrossRef]
- Ejike, C.E.; Collins, S.A.; Balasuriya, N.; Swanson, A.K.; Mason, B.; Udenigwe, C.C. Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci. Technol. 2017, 59, 30–36. [Google Scholar] [CrossRef]
- Udenigwe, C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Li-Chan, E.C. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Musoles, R.; Castello-Ruiz, M.; Arce, C.; Manzanares, P.; Ivorra, M.D.; Salom, J.B. Antihypertensive mechanism of lactoferrin-derived peptides: Angiotensin receptor blocking effect. J. Agric. Food Chem. 2014, 62, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Structure and activity study of egg protein ovotransferrin derived peptides (I.R.W. and I.Q.W.) on endothelial inflammatory response and oxidative stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef]
- Majumder, K.; Liang, G.; Chen, Y.; Guan, L.; Davidge, S.T.; Wu, J. Egg ovotransferrin-derived ACE inhibitory peptide I.R.W. increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 1735–1744. [Google Scholar] [CrossRef] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Majumder, K.; Li, Q.; Panahi, S.; Morton, J.S.; Davidge, S.T.; Wu, J. Egg white protein hydrolysate reduces blood pressure, improves vascular relaxation and modifies aortic angiotensin II receptors expression in spontaneously hypertensive rats. J. Funct. Food 2016, 27, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; Manso, M.; Aleixandre, A.; Alonso, M.J.; Salaices, M.; López-Fandiño, R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg white. J. Agric. Food Chem. 2007, 55, 10615–10621. [Google Scholar] [CrossRef] [PubMed]
- Shobako, N.; Ogawa, Y.; Ishikado, A.; Harada, K.; Kobayashi, E.; Suido, H.; Kusakari, T.; Maeda, M.; Suwa, M.; Matsumoto, M.; et al. A Novel Antihypertensive Peptide Identified in Thermolysin-Digested Rice Bran. Mol. Nutr. Food Res. 2018, 62, 1700732. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, J.; Pan, H.; Wu, H.; Ren, D.; Lu, J. Effects of I.Q.P., VEP and Spirulina platensis hydrolysates on the local kidney renin angiotensin system in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 16, 8485–8492. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Lafarga, T.; Aluko, R.E.; Rai, D.K.; O’Connor, P.; Hayes, M. Identification of bioactive peptides from a papain hydrolysate of bovine serum albumin and assessment of an antihypertensive effect in spontaneously hypertensive rats. Food Res. Int. 2016, 81, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, Y.; Zhang, Y.; Ruan, X.; Zhang, R. Purification, characterization, synthesis, in vitro ACE inhibition and in vivo antihypertensive activity of bioactive peptides derived from oil palm kernel glutelin-2 hydrolysates. J. Funct. Foods 2017, 28, 48–58. [Google Scholar] [CrossRef]
- Sánchez, D.; Kassan, M.; del Mar Contreras, M.; Carrón, R.; Recio, I.; Montero, M.-J.; Sevilla, M.-Á. Long-term intake of a milk casein hydrolysate attenuates the development of hypertension and involves cardiovascular benefits. Pharmacol. Res. 2011, 63, 398–404. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Antihypertensive effect of angiotensin-converting enzyme inhibitory peptide R.V.P.S.L. on spontaneously hypertensive rats by regulating gene expression of the Renin–Angiotensin System. J. Agric. Food Chem. 2014, 62, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Iwasaki, M.; Usui, H.; Ohinata, K.; Marczak, E.D.; Lipkowski, A.W.; Yoshikawa, M.J.P. Rapakinin, an antihypertensive peptide derived from rapeseed protein, dilates mesenteric artery of spontaneously hypertensive rats via the prostaglandin I.P. receptor followed by CCK1 receptor. Peptides 2010, 31, 909–914. [Google Scholar] [CrossRef]
- Aihara, K.; Osaka, M.; Yoshida, M. Oral administration of the milk casein-derived tripeptide Val-Pro-Pro attenuates high-fat diet-induced adipose tissue inflammation in mice. Br. J. Nutr. 2014, 112, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Girgih, A.T.; Alashi, A.M.; He, R.; Malomo, S.A.; Raj, P.; Netticadan, T.; Aluko, R.E. A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrients 2014, 6, 5652–5666. [Google Scholar] [CrossRef] [Green Version]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Nutrients 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Gu, L.; Ye, P.; Li, H.; Wang, Y.; Xu, Y.; Tian, Q.; Lei, G.; Zhao, C.; Gao, Z.; Zhao, W.; et al. Lunasin attenuates oxidant-induced endothelial injury and inhibits atherosclerotic plaque progression in ApoE−/− mice by up-regulating heme oxygenase-1 via PI3K/Akt/Nrf2/A.R.E. pathway. FASEB J. 2019, 33, 4836–4850. [Google Scholar] [CrossRef]
- Benkendorff, K.; Rudd, D.; Nongmaithem, B.D.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the traditional medical uses of Muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and characterization of bioactive peptides from soy hydrolysate and soy-fermented food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and anti-diabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo, J.M.; Munekata, P.E.; Gomez, B.; Barba, F.J.; Mora, L.; Perez-Santaescolastica, C.; Toldra, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R.; Kapila, S. Biofunctional properties of bioactive peptides of milk origin. Food Rev. Int. 2008, 25, 28–43. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.M.; Sakr, S.S.; El-Dieb, S.M.; Elkashef, H.A.S. Bioactive peptides with ACE-I and antioxidant activity produced from milk proteolysis. Int. J. Food Prop. 2017, 20, 3033–3042. [Google Scholar] [CrossRef] [Green Version]
- Mada, S.B.; Ugwu, C.P.; Abarshi, M.M. Health promoting effects of food-derived bioactive peptides: A review. Int. J. Pept. Res. Ther. 2019, 26, 831–848. [Google Scholar] [CrossRef]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro simulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Boga, S.; Bouzada, D.; Garcia Pena, D.; Vazquez Lopez, M.; Vázquez, M.E. Sequence-Specific DNA Recognition with Designed Peptides. Eur. J. Org. Chem. 2018, 2018, 249–261. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.; Santiago-López, L.; Peres, C.; Peres, C.; Garcia, H.; Vallejo-Cordoba, B.; González-Córdova, A.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- de Zani, S.C.; Wu, J.; Chan, C.B. Egg and soy-derived peptides and hydrolysates: A review of their physiological actions against diabetes and obesity. Nutrients 2018, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, S.M.; Gabaldón-Hernández, J.A.; Montoro-García, S. Unravelling the molecular mechanisms associated with the role of food-derived bioactive peptides in promoting cardiovascular health. J. Funct. Foods 2020, 64, 103645. [Google Scholar] [CrossRef]
- Giannetto, A.; Esposito, E.; Lanza, M.; Oliva, S.; Riolo, K.; Di Pietro, S.; Abbate, J.M.; Briguglio, G.; Cassata, G.; Cicero, L. Protein hydrolysates from anchovy (Engraulis encrasicolus) waste: In vitro and in vivo biological activities. Mar. Drugs 2020, 18, 86. [Google Scholar] [CrossRef]
- Lapphanichayakool, P.; Sutheerawattananonda, M.; Limpeanchob, N. Hypocholesterolemic effect of sericin-derived oligopeptides in high-cholesterol fed rats. J. Nat. Med. 2017, 71, 208–215. [Google Scholar] [CrossRef]
- Wang, J.; Shimada, M.; Kato, Y.; Kusada, M.; Nagaoka, S. Cholesterol-lowering effect of rice bran protein containing bile acid-binding proteins. Biosci. Biotechnol. Biochem. 2015, 79, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.R.; Freitas, R.A.M.S.; Carlos, A.C.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A.G. Peptides from cowpea present antioxidant activity, inhibit cholesterol synthesis and its solubilisation into micelles. Food Chem. 2015, 168, 288–293. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navab, M.; Anantharamaiah, G.; Reddy, S.T.; Hama, S.; Hough, G.; Frank, J.S.; Grijalva, V.R.; Ganesh, V.K.; Mishra, V.K.; Palgunachari, M.N.; et al. Oral small peptides render HDL anti-inflammatory in mice and monkeys and reduce atherosclerosis in ApoE null mice. Circ. Res. 2005, 97, 524–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed peptides exert hypocholesterolemic effects with a statin-like mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A. IAVPGEVA, IAVPTGVA, and LPYP, three peptides from soy glycinin, modulate cholesterol metabolism in HepG2 cells through the activation of the LDLR-SREBP2 pathway. Int. J. Mol. Sci. 2015, 14, 469–478. [Google Scholar] [CrossRef]
- Soares, R.A.M.; Mendonça, S.; De Castro, L.Í.A.; Menezes, A.C.C.C.C.; Arêas, J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015, 16, 4150–4160. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-H.; Chang, D.-K.; Chou, M.-J.; Huang, K.-J.; Shiuan, D. Peptide inhibitors of human HMG-CoA reductase as potential hypocholesterolemia agents. Biochem. Biophys. Res. Commun. 2015, 456, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory element binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the hypocholesterolaemic activity of LILPKHSDAD and LTFPGSAED, two peptides from lupin β-conglutin: Focus on L.D.L.R. and PCSK9 pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Calabresi, L.; Arnoldi, A. Lupin protein exerts cholesterol-lowering effects targeting PCSK9: From clinical evidences to elucidation of the in vitro molecular mechanism using HepG2 cells. J. Funct. Foods 2016, 23, 230–240. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Aiello, G.; Arnoldi, A.; Grazioso, G. Lupin peptides modulate the protein-protein interaction of PCSK9 with the low density lipoprotein receptor in HepG2 cells. Sci. Rep. 2016, 6, 29931. [Google Scholar] [CrossRef] [PubMed]
- Boachie, R.; Yao, S.; Udenigwe, C.C. Molecular mechanisms of cholesterol-lowering peptides derived from food proteins. Curr. Opin. Food Sci. 2018, 20, 58–63. [Google Scholar] [CrossRef]
- Moore, K.J.; Rayner, K.J.; Suárez, Y.; Fernández-Hernando, C. microRNAs and cholesterol metabolism. Trends Endocrinol. Metab. 2010, 21, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Marquart, T.J.; Allen, R.M.; Ory, D.S.; Baldán, Á. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 12228–12232. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Betters, J.L.; Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Avraham, S.; Harman-Boehm, I.; Schwarzfuchs, D.; Shai, I. Dietary strategies for patients with type 2 diabetes in the era of multi-approaches; review and results from the Dietary Intervention Randomized Controlled Trial (DIRECT). DIABETES Res. Clin. Pract. 2009, 86, S41–S48. [Google Scholar] [CrossRef]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- Lebovitz, H.E.J.E.; Clinics, M. Oral therapies for diabetic hyperglycemia. Endocrinol. Metab. Clin. 2001, 30, 909–933. [Google Scholar] [CrossRef]
- Masilamani, M.; Wei, J.; Sampson, H.A. regulation of the immune response by soybean isoflavones. Immunol. Res. 2012, 54, 95–110. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Pokora, M.; Setner, B.; Dąbrowska, A.; Szołtysik, M.; Babij, K.; Szewczuk, Z.; Trziszka, T.; Lubec, G.; Chrzanowska, J. Multifunctional peptides derived from an egg yolk protein hydrolysate: Isolation and characterization. Amino Acids 2015, 47, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, M.; Kuroda, T.; Matsuo, T. Increased muscular triglyceride content and hyperglycemia in Goto-Kakizaki rat are decreased by egg white hydrolysate. Int. J. Food Sci. Nutr. 2014, 65, 495–501. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Miyamoto, Y.; Shibata, Y.; Yoshimura, K.; Izumida, E.; Suzuki, H.; Miyazaki, T.; Maki, K.; Kamijo, R. In situ quasi-static and dynamic nanoindentation tests on calcified nodules formed by osteoblasts: Implication of glucocorticoids responsible for osteoblast calcification. Acta Biomater. 2015, 12, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, M.; Yadav, D. Food bioprocessing by non-thermal plasma technology. Curr. Opin. Food Sci. 2018, 19, 85–91. [Google Scholar] [CrossRef]
- Nilsson, E.; Millberg, M.; Oberg, J.; Jantsch, A. Load Distribution with the Proximity Congestion Awareness in A Network on Chip. In Proceedings of the 2003 Design, Automation and Test in Europe Conference and Exhibition, Munich, Germany, 7 March 2003; pp. 1126–1127. [Google Scholar]
- Sun, M.; Gadad, S.S.; Kim, D.-S.; Kraus, W.L. Discovery, annotation, and functional analysis of long noncoding R.N.A.s controlling cell-cycle gene expression and proliferation in breast cancer cells. Mol. Cell 2015, 59, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Dziuba, B.; Dziuba, M. Milk proteins-derived bioactive peptides in dairy products: Molecular, biological and methodological aspects. Acta Sci. Pol. Technol. Aliment. 2014, 13, 5–26. [Google Scholar] [CrossRef]
- Chum, H.; Johnson, D.; Black, S.; Baker, J.; Grohmann, K.; Sarkanen, K.; Wallace, K.; Schroeder, H. Organosolv pretreatment for enzymatic hydrolysis of poplars: I. Enzyme hydrolysis of cellulosic residues. Biotechnol. Bioeng. 1988, 31, 643–649. [Google Scholar] [CrossRef]
- El-Zawawy, W.K.; Ibrahim, M.M.; Abdel-Fattah, Y.R.; Soliman, N.A.; Mahmoud, M.M. Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr. Polym. 2011, 84, 865–871. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Takano, T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J. Dairy Sci. 1995, 78, 1253–1257. [Google Scholar] [CrossRef]
- Rokka, T.; Syväoja, E.-L.; Tuominen, J.; Korhonen, H.J. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus G.G. fermented UHT-milk. Milchwiss. Milk Sci. Int. 1997, 52, 675–678. [Google Scholar]
- Maeno, M.; Yamamoto, N.; Takano, T. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996, 79, 1316–1321. [Google Scholar] [CrossRef]
- Yamamoto, N.; Takano, T.J.F.N. Antihypertensive peptides derived from milk proteins. Food/Nahrung 1999, 43, 159–164. [Google Scholar] [CrossRef]
- Gobbetti, M.; Stepaniak, L.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Latent bioactive peptides in milk proteins: Proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 2002, 42, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, Y.; Matsuda, S.; Igoshi, K.; Oki, T. Antioxidative peptide from milk fermented with Lactobacillus delbrueckii subsp. bulgaricus IFO13953. Nippon. Shokuhin Kagaku Kogaku Kaishi 2001, 48, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ledesma, B.; Amigo, L.; Ramos, M.; Recio, I. Angiotensin converting enzyme inhibitory activity in commercial fermented products. Formation of peptides under simulated gastrointestinal digestion. J. Agric. Food Chem. 2004, 52, 1504–1510. [Google Scholar] [PubMed]
- Chand, R.; Ashar, M.N. Fermented milk containing ACE-inhibitory peptides reduces blood pressure in middle aged hypertensive subjects. Milchwissenschaft 2004, 59, 363–366. [Google Scholar]
- Ochiai, M.; Matsuo, T. Effect of egg white and its hydrolysate on stearoyl-CoA desaturase index and fat accumulation in rat tissues. Int. J. Food Sci. Nutr. 2014, 65, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Garcés-Rimón, M.; López-Expósito, I.; López-Fandiño, R.; Miguel, M. Egg white hydrolysates with in vitro biological multiactivities to control complications associated with the metabolic syndrome. Eur. Food Res. Technol. 2016, 242, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Garcés-Rimón, M.; González, C.; Uranga, J.; López-Miranda, V.; López-Fandiño, R.; Miguel, M. Pepsin egg white hydrolysate ameliorates obesity-related oxidative stress, inflammation and steatosis in Zucker fatty rats. PLoS ONE 2016, 11, e0151193. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, M.; Misaki, K.; Takeuchi, T.; Narumi, R.; Azuma, Y.; Matsuo, T. Egg white hydrolysate can be a low-allergenic food material to suppress ectopic fat accumulation in rats fed an equicaloric diet. J. Nutr. Sci. Vitaminol. 2017, 63, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Requena, T.; Miguel, M.; Garcés-Rimón, M.; Martínez-Cuesta, M.C.; López-Fandiño, R.; Peláez, C. Pepsin egg white hydrolysate modulates gut microbiota in Zucker obese rats. Food Funct. 2017, 8, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow K.K.). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Kwak, J.H.; Ahn, C.-W.; Park, S.-H.; Jung, S.-U.; Min, B.-J.; Kim, O.Y.; Lee, J.H. Weight reduction effects of a black soy peptide supplement in overweight and obese subjects: Double blind, randomized, controlled study. Food Funct. 2012, 3, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baer, I.; De La Calle, B.; Taylor, P. 3-MCPD in food other than soy sauce or hydrolysed vegetable protein (H.V.P.). Anal. Bioanal. Chem. 2010, 396, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.C.O.; Tironi, V.A.; Añón, M.C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. LWT Food Sci. Technol. 2011, 44, 1752–1760. [Google Scholar] [CrossRef]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Whitehead, R.; Gardner, J. In vitro growth of epithelial cells from mucosa derived from different regions of the gastrointestinal tract. J. Gastroenterol. Hepatol. 1987, 2, 59–66. [Google Scholar] [CrossRef]
- Parker, C.S.; Topol, J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell 1984, 37, 273–283. [Google Scholar] [CrossRef]
- Calfee, D.P.; Salgado, C.D.; Classen, D.; Arias, K.M.; Podgorny, K.; Anderson, D.J.; Burstin, H.; Coffin, S.E.; Dubberke, E.R.; Fraser, V. Strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect. Control. Hosp. Epidemiol. 2008, 29, S62–S80. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; De Biase, D.; Severini, C.; Aita, M.; Erspamer, G.F.; Barra, D.; Bossa, F. Purification and characterization of bioactive peptides from skin extracts of Rana esculenta. Biochim. Biophys. Acta (BBA) Gen. Subj. 1990, 1033, 318–323. [Google Scholar] [CrossRef]
- Mekata, T.; Kono, T.; Satoh, J.; Yoshida, M.; Mori, K.; Sato, T.; Miyazato, M.; Ida, T. Purification and characterization of bioactive peptides RYamide and CCHamide in the kuruma shrimp Marsupenaeus japonicus. Gen. Comp. Endocrinol. 2017, 246, 321–330. [Google Scholar] [CrossRef]










| Peptide | Source | Animal | Activity | Reference |
|---|---|---|---|---|
| Peptides with antihypertensive effects: RRWQWR, IJWKL and RPYL | Milk, Derived from lactoferrin | SHRs | Reduces Ang II-induced vasoconstriction in isolated rabbit carotid arterial segments. | [11] |
| IRW | Derived from egg ovotransferrin | SHRs | Dose-dependently attenuates BP by ~10 mmHg and ~40 mmHg in the low- and high-dose groups, respectively, compared with the results obtained with untreated SHRs. Increases the expression of ACE-2, ATP-binding cassette subfamily B member 1, interferon-regulatory factor and cadherin 1 while significantly decreasing the expression of ICAM-1 and VCAM-1 in mesenteric arteries. | [12,13] |
| EWH (egg white protein hydrolysate) | Egg white protein | SHRs | Improves vascular relaxation and modifies aortic Ang II receptor expression. | [14] |
| YRGGLEPINF ESIINF | Egg white protein | SHRs | Its vascular-relaxing mechanism is independent of ACE inhibition. | [15] |
| TRB (Thermolysin- digested rice bran) LRA, YY | Thermolysin-digested rice bran | SHRs | The long-term administration of TRB (50 mg kg−1 d−1) lowers systolic blood pressure compared with that of the control group. TRB reduces ACE activity in the lung in a dose-dependent manner but does not affect ACE activities in the aorta, kidney, and heart tissues. LRA (0.25 mg kg − 1) and YY (0.5 mg kg − 1) lowers blood pressure 4 h after oral administration. | [16] |
| IQP, VEP | Spirulina platensis hydrolysates | SHRs | Inhibits ACE, Ang II and AT1R. Upregulates ACE2, Ang (1–7), Mas and AT2. | [17] |
| Salmon gelatine hydrolysate | Salmon | SHRs | ACE and DPP-IV inhibitory activities in vitro. | [18] |
| SLR, YY, ER, and FR | Papain-digested bovine serum albumin | SHRs | ACE inhibitory activity in vitro and in vivo. | [19] |
| Hydrolysate fraction | Palm kernel | SHRs | Antihypertensive effects. | [20] |
| Milk peptides | Milk protein hydrolysate | SHRs | Attenuate the development of hypertension: systolic blood pressure is increased 33 ± 3 mmHg in the control group compared to 18 ± 5 mmHg in the treated group. Improve aorta and mesenteric acetylcholine relaxation. Increase eNOS expression in the aorta. Decrease left ventricular hypertrophy and interstitial fibrosis. | [21] |
| RVPSL | Egg | SHRs | Dose-dependently decreases systolic blood pressure starting one week after the administration of a maximum dose of 50 mg/kg. Increases the mRNA expression of renin, ACE, and AT1 receptor in the kidney. Decreases the serum Ang II, renin, and aldosterone levels. | [22] |
| Rapakinin (RIY) | Rapeseed | SHRs | Induces dilatation of mesenteric artery mediated mainly by the PGI2–IP receptor and then CCK–CCK1 receptor-dependent vasorelaxation. | [23] |
| Other effects VPP | Milk, casein-derived | CSJEL/61 Mice | Attenuates high-fat diet-induced adipose tissue inflammation. | [24] |
| Hemp seed Protein hydrolysate | Hemp seed | SHRs | Decreases SOD and catalase expression and the total peroxides levels. | [25] |
| Milk peptides | Fermented milk with Lactococcus lactis NRRL B-50571 | SHRs | Enhance nitric oxide production and antioxidant activity. | [26] |
| Lunasin | Soy | Apolipoprotein E–deficient (ApoE-/-) mice | Decreases plaque formation in an experimental ApoE2/2 atherosclerotic model. | [27] |
| Peptide Source | Peptide Sequence | Hypocholesterolaemic Mechanism | Reference |
|---|---|---|---|
| Cowpea | Peptide mixtures | Binding to bile acids/salts or lipids and inhibition of micellar cholesterol solubility | [42,43,44,45,46] |
| Sericin | Peptide mixtures | ||
| Royal jelly | Peptide mixtures | ||
| Chemical synthesis | KRES | ||
| Rice bran | Not applicable | ||
| Cowpea | Peptide mixtures | ||
| Lupin | Peptide mixtures | ||
| Hempseed | Peptide mixtures | ||
| Amaranth | GGV, IVG, VGVL | ||
| Soy b-conglycinin | YVVNPDNDEN | Inhibition of HMGCoAR activity and inhibition of the mevalonate pathway and cholesterol biosynthesis | [44,47,48,49,50,51] |
| Soy b-conglycinin | YVVNPDNDEN | ||
| Soy glycinin | IAVPGEVA | ||
| Soy glycinin | IAVPTGVA | ||
| Soy glycinin | LPYP | ||
| Lupin b-conglutin | LILPKHSDAD | ||
| Lupin b-conglutin | LTFPGSAED | ||
| Chemical synthesis | P.M.A.S. | ||
| Lupin | Peptide mixtures | ||
| Hempseed | Peptide mixtures | ||
| Soy glycinin | IAVPGEVA | Increases in the SREBP2 and L.D.L.R. protein levels and increases in LDL uptake and cholesterol degradation | [47,48,52] |
| Soy glycinin | IAVPTGVA | ||
| Soy glycinin | LPY.P. | ||
| Soy b-conglycinin | YVVNPDNDEN | ||
| Soy b-conglycinin | YVVNPDNDEN | ||
| Lupin proteins | Peptide mixtures | Decreases in PCSK9 production (via an effect on HNF1a protein) and secretion and increases in the LDLR level and the uptake of LDL by hepatocytes | [53,54] |
| Lupin b-conglutin | LILPKHSDAD | Inhibition of PCSK9–L.D.L.R. interaction and increase in LDL uptake | [55,56] |
| Antidiabetic Peptides | Hypoglycaemia | Weight Gain | Oedema | GI Effects | Lactic Acidosis | Liver Toxicity |
|---|---|---|---|---|---|---|
| Glipizide XL | 1+ | 1+ | 0 | ± | 0 | ± |
| Glyburide | 4+ | 2+ | 0 | ± | 0 | ± |
| Glimepiride | 2+ | 1+ | 0 | ± | 0 | ± |
| Repaglinide | 1+ | 1+ | 0 | 0 | 0 | 0 |
| Nateglinide | 1+ | ↓ | 0 | 0 | 0 | 0 |
| Metformin | 0 | ? | 0 | 2+ | 1+ | 0 |
| Acarbose | 0 | 0 | 0 | 3+ | 0 | ± |
| Miglitol | 0 | 0 | 0 | 3+ | 0 | 0 |
| Rosiglitazone | 0 | 3+ | 2+ | 0 | 0 | 0 * |
| Pioglitazone | 0 | 3+ | 2+ | 0 | 0 | 0 * |
| Microorganisms Used | Precursor Protein a | Peptide Sequence | Bioactivity | References |
|---|---|---|---|---|
| Lactobacillus helveticus, Sacch aromyces cerevisiae | β-cn, k-cn | Val-Pro-Pro, Ile-Pro-Pro | ACE inhibitory and antihypertension activities | [74,75] |
| Lactobacillus G.G. enzymes + pepsin and trypsin | β-cn, αs1-cn | Tyr-Pro-Phe-Pro, Ala-Val-Pro-Tyr-Pro-Gln-Arg, Thr-Thr-Met-Pro-Leu-Trp | Opioid and ACE inhibitory activity immunostimulatory activity | [76] |
| L. helveticus CP90 proteinase | β-cn | Tyr-Pro-Phe-Pro, Ala-Val-Pro-Tyr-Pro-Gln-Arg, Thr-Thr-Met-Pro-Leu-Trp | ACE inhibitory activity | [77] |
| L. helveticus CPN 4 | Whey proteins | Tyr-Pro | ACE inhibitory activity | [78] |
| L. delbrueckii subsp. bulgaricus SS1, Lactococcus lactis subsp. cremoris FT4 | β-cn, k-cn | Many fragments | ACE inhibitory activity | [79] |
| L. delbrueckii subsp. bulgaricus IFO13953 | k-cn | Ala-Arg-His-Pro-His-Pro-His-Leu-Ser-Phe-Met | Antioxidative activity | [80] |
| L. rhamnosus + digestion with pepsin and Corolase PP | β-cn | Asp-Lys-Ile-His-Pro-Phe, Tyr-Gln-Glu-Pro-Val-Leu Val-Lys-Glu-Ala-Met-Ala-Pro-Lys | ACE inhibitory activity Antioxidative activity | [81] |
| L. delbrueckii subsp. bulgaricus | β-cn | Ser-Lys-Val-Tyr-Pro-Phe-Pro-Gly-Pro-Ile | ACE inhibitory activity | [82] |
| Streptococcus thermophilus + L. lactis subsp. lactis biovar. diacetylactis | β-cn | Ser-Lys-Val-Tyr-Pro | ACE inhibitory activity | [82] |
| L. helveticus ICM 1004 cell-free extract | Skim milk hydrolysate | Val-Pro-Pro, Ile-Pro-Pro | ACE inhibitory activity | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, R.; Wei, S.; Daliri, E.B.-M.; Elahi, F.; Yeon, S.-J.; Tyagi, A.; Liu, S.; Madar, I.H.; Sultan, G.; Oh, D.-H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods 2021, 10, 2220. https://doi.org/10.3390/foods10092220
Chelliah R, Wei S, Daliri EB-M, Elahi F, Yeon S-J, Tyagi A, Liu S, Madar IH, Sultan G, Oh D-H. The Role of Bioactive Peptides in Diabetes and Obesity. Foods. 2021; 10(9):2220. https://doi.org/10.3390/foods10092220
Chicago/Turabian StyleChelliah, Ramachandran, Shuai Wei, Eric Banan-Mwine Daliri, Fazle Elahi, Su-Jung Yeon, Akanksha Tyagi, Shucheng Liu, Inamul Hasan Madar, Ghazala Sultan, and Deog-Hwan Oh. 2021. "The Role of Bioactive Peptides in Diabetes and Obesity" Foods 10, no. 9: 2220. https://doi.org/10.3390/foods10092220