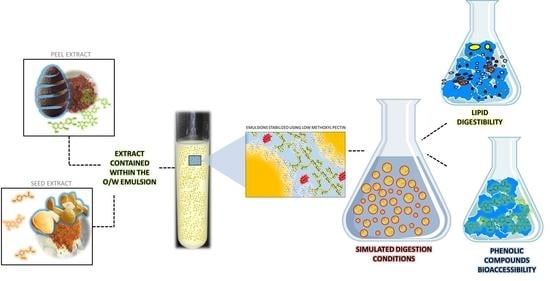
Lipid Digestibility and Polyphenols Bioaccessibility of Oil-in-Water Emulsions Containing Avocado Peel and Seed Extracts as Affected by the Presence of Low Methoxyl Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Avocado Peel and Seed Extracts
2.2. Identification and Tentative Quantification of Individual Phenolic Compounds
2.3. Emulsion’s Formation
2.4. Emulsion Droplet Size and Droplet Size Distribution
2.5. Emulsion In Vitro Digestibility
2.6. Bioaccessibility of Phenolic Compounds
2.7. Statistical Analysis
3. Results and Discussions
3.1. Tentative Quantification of the Individual Phenolic Compounds in Avocado Peel and Seed Extracts by UPLC-ESI-MS/MS
3.2. Effect of Avocado Peel and Seed Extracts on the Formation of O/W Emulsions Using LMP as Surfactant
3.3. Effect of LMP on the Digestibility of Emulsions
3.3.1. Colloidal Stability of Emulsions during Digestion
3.3.2. Lipid Digestibility
3.3.3. Phenolic Compounds Bioaccessibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; González-Aguilar, G.A.; Martín-Belloso, O. Interfacial activity of phenolic-rich extracts from avocado fruit waste: Influence on the colloidal and oxidative stability of emulsions and nanoemulsions. Innov. Food Sci. Emerg. Technol. 2021, 69, 102665. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids—Food Sources, Health Benefits, and Mechanisms Involved. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Gewerbestrasse, Cham, Switzerland, 2017; pp. 1–27. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Quero, J.; Osada, J.; Martín-Belloso, O.; Rodríguez-Yoldi, M.J. Phenolic-Rich Extracts from Avocado Fruit Residues as Functional Food Ingredients with Antioxidant and Antiproliferative Properties. Biomolecules 2021, 11, 977. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Quiles-Chuliá, A.; Martín-Belloso, O.; Hernando-Hernando, I.; Soliva-Fortuny, R. Effect of pulsed electric fields on carotenoid and phenolic bioaccessibility and their relationship with carrot structure. Food Funct. 2021, 12, 2772–2783. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Assadpour, E.; Jafari, S.M. Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.; Wang, K.; Zhu, X.; Chen, D.; Zhuang, H.; Yao, L.; Song, S.; Wang, H.; Sun, M. Emulsion-based delivery systems for curcumin: Encapsulation and interaction mechanism between debranched starch and curcumin. Int. J. Biol. Macromol. 2020, 161, 746–754. [Google Scholar] [CrossRef]
- Mitsou, E.; Dupin, A.; Sassi, A.H.; Monteil, J.; Sotiroudis, G.T.; Leal-Calderon, F.; Xenakis, A. Hydroxytyrosol encapsulated in biocompatible water-in-oil microemulsions: How the structure affects in vitro absorption. Colloids Surf. B Biointerfaces 2019, 184, 110482. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: A review. Food Hydrocoll. 2021, 118, 106772. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Oliveira, A.L.; von Staszewski, M.; Pizones Ruiz-Henestrosa, V.M.; Pintado, M.; Pilosof, A.M.R. Impact of pectin or chitosan on bulk, interfacial and antioxidant properties of (+)-catechin and β-lactoglobulin ternary mixtures. Food Hydrocoll. 2016, 55, 119–127. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Martín-Belloso, O. Influence of mandarin fiber addition on physico-chemical properties of nanoemulsions containing β-carotene under simulated gastrointestinal digestion conditions. LWT 2017, 84, 331–337. [Google Scholar] [CrossRef]
- Aguilera-Angel, E.-Y.; Espinal-Ruiz, M.; Narváez-Cuenca, C.-E. Pectic polysaccharides with different structural characteristics as inhibitors of pancreatic lipase. Food Hydrocoll. 2018, 83, 229–238. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Kent, K.; Charlton, K.; O’Sullivan, T.; Oddy, W.H. Estimated intake and major food sources of flavonoids among Australian adolescents. Eur. J. Nutr. 2020, 59, 1–16. [Google Scholar] [CrossRef]
- Yamagata, K.; Yamori, Y. Inhibition of endothelial dysfunction by dietary flavonoids and preventive effects against cardiovascular disease. J. Cardiovasc. Pharmacol. 2020, 75, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Lu, Z.; Jia, Q.; Wang, R.; Wu, X.; Wu, Y.; Huang, C.; Li, Y. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine 2011, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Kumar, K.; Issac, A.; Ninan, E.; Kuttan, R.; Maliakel, B. Enhanced anti-diabetic activity of polyphenol-rich de-coumarinated extracts of Cinnamomum cassia. J. Funct. Foods 2014, 10, 54–64. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Escalona-Buendía, H.B.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Pedroza-Islas, R.; Ponce-Alquicira, E. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT–Food Sci. Technol. 2016, 65, 46–52. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crop. Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, M.; Merinen, M.; Kilpeläinen, P.O.; Xu, C.; Willför, S.M.; Mikkonen, K.S. Phenolic residues in spruce galactoglucomannans improve stabilization of oil-in-water emulsions. J. Colloid Interface Sci. 2018, 512, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Di Mattia, C.; Paradiso, V.M.; Andrich, L.; Giarnetti, M.; Caponio, F.; Pittia, P. Effect of Olive Oil Phenolic Compounds and Maltodextrins on the Physical Properties and Oxidative Stability of Olive Oil O/W Emulsions. Food Biophys. 2014, 9, 396–405. [Google Scholar] [CrossRef]
- Di Mattia, C.; Sacchetti, G.; Mastrocola, D.; Sarker, D.; Pittia, P. Surface properties of phenolic compounds and their influence on the dispersion degree and oxidative stability of olive oil O/W emulsions. Food Hydrocoll. 2010, 24, 652–658. [Google Scholar] [CrossRef]
- Giacintucci, V.; Di Mattia, C.; Sacchetti, G.; Neri, L.; Pittia, P. Role of olive oil phenolics in physical properties and stability of mayonnaise-like emulsions. Food Chem. 2016, 213, 369–377. [Google Scholar] [CrossRef]
- Michalík, M.; Poliak, P.; Klein, E.; Lukeš, V. On the toxicity of para-substituted phenols and their quinone metabolites: Quantum chemical study. Chem. Phys. Lett. 2018, 709, 71–76. [Google Scholar] [CrossRef]
- Koh, J.; Xu, Z.; Wicker, L. Binding kinetics of blueberry pectin-anthocyanins and stabilization by non-covalent interactions. Food Hydrocoll. 2020, 99, 105354. [Google Scholar] [CrossRef]
- Wijaya, W.; Zheng, H.; Zheng, T.; Su, S.; Patel, A.R.; Van der Meeren, P.; Huang, Q. Improved bioaccessibility of polymethoxyflavones loaded into high internal phase emulsions stabilized by biopolymeric complexes: A dynamic digestion study via TNO’s gastrointestinal model. Curr. Res. Food Sci. 2020, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-Z.; Wu, X.-Z.; Pan, L.-H.; Zheng, Z.; Zhang, M. Pectin-peptide complexes ameliorated physicochemical stabilities and in vitro digestion abilities of β-carotene loaded emulsions. Food Chem. 2021, 340, 128209. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Influence of particle size on lipid digestion and β-carotene bioaccessibility in emulsions and nanoemulsions. Food Chem. 2013, 141, 1472–1480. [Google Scholar] [CrossRef]
- Santiago, J.S.J.; Salvia-Trujillo, L.; Zucca, R.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. In vitro digestibility kinetics of oil-in-water emulsions structured by water-soluble pectin-protein mixtures from vegetable purées. Food Hydrocoll. 2018, 80, 231–244. [Google Scholar] [CrossRef]
- Miao, J.; Xu, N.; Cheng, C.; Zou, L.; Chen, J.; Wang, Y.; Liang, R.; McClements, D.J.; Liu, W. Fabrication of polysaccharide-based high internal phase emulsion gels: Enhancement of curcumin stability and bioaccessibility. Food Hydrocoll. 2021, 117, 106679. [Google Scholar] [CrossRef]
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; de Souza, F.G.; Catharino, R.R.; do Sacramento, C.K.; Pastore, G.M. Gastrointestinal bioaccessibility and bioactivity of phenolic compounds from araçá-boi fruit. LWT 2021, 135, 110230. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Ismail, B.B.; Guo, M.; Pu, Y.; Çavuş, O.; Ayub, K.A.; Watharkar, R.B.; Ding, T.; Chen, J.; Liu, D. Investigating the effect of in vitro gastrointestinal digestion on the stability, bioaccessibility, and biological activities of baobab (Adansonia digitata) fruit polyphenolics. LWT 2021, 145, 111348. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, B.; Xia, Y.; Li, H.; Shi, X.; Wang, J.; Deng, Z. Bioaccessibility and transformation pathways of phenolic compounds in processed mulberry (Morus alba L.) leaves after in vitro gastrointestinal digestion and faecal fermentation. J. Funct. Foods 2019, 60, 103406. [Google Scholar] [CrossRef]
- Ribeiro, L.d.O.; Pinheiro, A.C.B.; Brígida, A.I.S.; Genisheva, Z.A.; Vicente, A.A.M.d.O.S.; Teixeira, J.A.C.; de Matta, V.M.; Freitas, S.P. In vitro gastrointestinal evaluation of a juçara-based smoothie: Effect of processing on phenolic compounds bioaccessibility. J. Food Sci. Technol. 2019, 56, 5017–5026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, M.; Rocchetti, G.; Ghisoni, S.; Giuberti, G.; Capanoglu, E.; Lucini, L. Effect of different soluble dietary fibres on the phenolic profile of blackberry puree subjected to in vitro gastrointestinal digestion and large intestine fermentation. Food Res. Int. 2020, 130, 108954. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Time (min) | Acetic Acid (%) | Acetonitrile (%) |
|---|---|---|
| 0 | 95 | 5 |
| 5 | 90 | 10 |
| 10 | 87.6 | 12.4 |
| 18 | 72 | 28 |
| 23 | 0 | 100 |
| 25.5 | 0 | 100 |
| 27 | 95 | 5 |
| 30 | 95 | 5 |
| No. | Phenolic Compound | MW (g/mol) | SRM Quantification | Cone Voltage (V) | Collision Energy (eV) | Standard in Which Has Been (Tentatively) Quantified | Peel | Seed |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | 1111.54 ± 11.25 | 377.98 ± 111.64 | ||||||
| 1 | p-hydroxybenzoic acid | 138 | 137 > 93 | 30 | 15 | p-Hydroxybenzoic Acid | 0.39 ± 0.02 | 0.12 ± 0.01 |
| 2 | Vanillin | 152 | 151 > 136 | 25 | 10 | Caffeic Acid | 0.10 ± 0.02 | 0.02 ± 0.01 |
| 3 | Vanillic acid | 168 | 167 > 123 | 30 | 10 | Caffeic Acid | 0.03 ± 0.00 | n.d. |
| 4 | Syringic acid | 198 | 197 > 182 | 30 | 10 | Caffeic Acid | 0.09 ± 0.02 | n.d. |
| 5 | Protocatechuic acid | 154 | 153 > 109 | 40 | 15 | Protocatehuic Acid | 2.20 ± 0.24 | 0.28 ± 0.13 |
| 6 | Protocatechuic acid glucoside | 316 | 315 > 153 | 40 | 20 | Protocatehuic Acid | 26.1 ± 0.27 | 1.95 ± 0.51 |
| 7 | Hydroxytyrosol | 154 | 153 > 123 | 35 | 10 | Hydroxytyrosol | 0.19 ± 0.09 | 0.18 ± 0.06 |
| 8 | Hydroxytyrosol glucoside | 316 | 315 > 153 | 40 | 20 | Hydroxytyrosol | 0.14 ± 0.01 | 0.91 ± 0.23 |
| 9 | Hydroxysalidroside | 316 | 315 > 135 | 40 | 30 | Hydroxytyrosol | 0.10 ± 0.02 | 5.30 ± 1.08 |
| 10 | Hydroxytyrosol glucoside arabinoside | 448 | 447 > 153 | 40 | 20 | Hydroxytyrosol | 2.54 ± 0.11 | n.d. |
| 11 | Tyrosol glucoside | 300 | 299 > 137 | 40 | 20 | Tyrosol | 8.30 ± 0.52 | 10.40 ± 2.70 |
| 12 | Salidroside | 300 | 299 > 179 | 40 | 10 | Tyrosol | 0.54 ± 0.10 | 148.40 ± 50.30 |
| 13 | Tyrosol glucoside arabinoside | 432 | 431 > 137 | 40 | 20 | Tyrosol | 62.9 ± 1.09 | 0.75 ± 0.30 |
| 14 | p-coumaric acid | 164 | 163 > 119 | 35 | 10 | p-Cumaric Acid | 0.25 ± 0.01 | 0.20 ± 0.02 |
| 15 | Coumaric acid glucoside | 326 | 325 > 163 | 40 | 20 | p-Cumaric Acid | 0.33 ± 0.00 | n.d. |
| 16 | Coumaroylquinic acid | 338 | 337 > 191 | 40 | 20 | p-Cumaric Acid | 1.38 ± 0.06 | 3.61 ± 1.35 |
| 17 | Caffeic acid | 180 | 179 > 135 | 35 | 15 | Caffeic Acid | 0.42 ± 0.11 | 0.59 ± 0.14 |
| 18 | Caffeic acid glucoside | 342 | 341 > 179 | 40 | 20 | Caffeic Acid | 0.37 ± 0.06 | 0.09 ± 0.01 |
| 19 | Caffeic acid glucoside derivative | 546 | 545 > 341 | 40 | 20 | Caffeic Acid | 0.08 ± 0.01 | n.d. |
| 20 | Dihydrocaffeic acid glucoside | 344 | 343 > 181 | 40 | 20 | Caffeic Acid | 0.08 ± 0.00 | 0.21 ± 0.04 |
| 21 | Caffeoylshikimic acid | 336 | 335 > 161 | 40 | 20 | Caffeic Acid | 0.27 ± 0.01 | 2.21 ± 0.77 |
| 22 | 3-O-caffeoylquinic acid | 354 | 353 > 179 | 40 | 15 | 5-O-Caffeoylquinic Acid | 17.60 ± 0.26 | 176.20 ± 53.40 |
| 23 | 4-O-caffeoylquinic acid | 354 | 353 > 173 | 40 | 15 | 5-O-Caffeoylquinic Acid | 9.56 ± 0.07 | 10.70 ± 4.12 |
| 24 | 5-O-caffeoylquinic acid | 354 | 353 > 191 | 40 | 15 | 5-O-Caffeoylquinic Acid | 969.20 ± 9.21 | 11.30 ± 4.26 |
| 25 | Dicaffeoylquinic acid | 516 | 515 > 191 | 40 | 30 | 5-O-Caffeoylquinic Acid | 1.05 ± 0.01 | n.d. |
| 26 | Ferulic acid | 194 | 193 > 134 | 30 | 15 | Ferulic Acid | 0.25 ± 0.01 | 0.06 ± 0.01 |
| 27 | Ferulic acid glucoside | 356 | 355 > 193 | 40 | 20 | Ferulic Acid | 4.14 ± 0.12 | 1.52 ± 0.56 |
| 28 | Dihydroferulic acid glucoside | 358 | 357 > 195 | 40 | 20 | Ferulic Acid | 0.24 ± 0.01 | 0.02 ± 0.00 |
| 29 | 4-O-feruoylquinic acid | 368 | 367 > 173 | 40 | 20 | Ferulic Acid | 0.49 ± 0.05 | 0.22 ± 0.06 |
| 30 | 5-O-feruoylquinic acid | 368 | 367 > 191 | 40 | 15 | Ferulic Acid | 1.61 ± 0.06 | 0.12 ± 0.6 |
| 31 | 3-O-feruoylquinic acid | 368 | 367 > 193 | 40 | 15 | Ferulic Acid | 0.76 ± 0.03 | 2.83 ± 1.57 |
| Flavonoids | 5721.88 ± 51.73 | 1135.77 ± 456.57 | ||||||
| 32 | Catechin | 290 | 289 > 245 | 40 | 15 | Catechin | n.d. | 280.50 ± 148.80 |
| 33 | Epicatechin | 290 | 289 > 245 | 40 | 15 | Epicatechin | 1891.00 ± 75.70 | 360.0 ± 140.60 |
| 34 | Catechin glucoside | 452 | 451 > 289 | 40 | 25 | Catechin | 2.40 ± 0.04 | 3.85 ± 0.08 |
| 35 | Epicatechin glucoside | 452 | 451 > 289 | 40 | 25 | Epicatechin | 7.89 ± 0.16 | 4.81 ± 0.66 |
| 36 | Epigallocatechin | 306 | 305 > 125 | 40 | 15 | Epicatechin | 6.27 ± 0.29 | 1.86 ± 0.67 |
| 37 | Epicatechin gallate | 442 | 441 > 169 | 40 | 20 | Epicatechin | n.d. | 1.39 ± 0.36 |
| 38 | Catechin derivative | 740 | 739 > 289 | 40 | 30 | Catechin | 1.55 ± 0.33 | 2.32 ± 0.27 |
| 39 | Epicatechin derivative | 740 | 739 > 289 | 40 | 30 | Epicatechin | 67.3 ± 9.96 | 1.72 ± 0.17 |
| 40 | Dimer (type A) | 576 | 575 > 285 | 40 | 20 | Dimer B2 | 4.45 ± 0.09 | 6.28 ± 8.88 |
| 41 | Dimer (type B) | 578 | 577 > 289 | 40 | 20 | Dimer B2 | 2262.0 ± 63.00 | 207.7 ± 112.10 |
| 42 | Trimer (type A) | 864 | 863 > 411 | 40 | 30 | Dimer B2 | 9.27 ± 1.21 | 231.4 ± 74.20 |
| 43 | Trimer (type B) | 866 | 865 > 287 | 60 | 30 | Dimer B2 | 383.2 ± 14.90 | 11.6 ± 16.50 |
| 44 | Tetramer | 1154 | 1153 > 865 | 70 | 20 | Dimer B2 | 106.20 ± 4.18 | 9.73 ± 2.91 |
| 45 | Pentamer | 1442 | 1441 > 1028 | 80 | 25 | Dimer B2 | 1.09 ± 0.06 | 0.55 ± 0.12 |
| 46 | Hexamer | 1730 | 1729 > 1153 | 80 | 30 | Dimer B2 | 6.80 ± 0.02 | 1.31 ± 0.15 |
| 47 | Quercetin | 302 | 301 > 151 | 40 | 15 | Quercetin-3-O-Glucoside | 0.70 ± 0.02 | 0.39 ± 0.00 |
| 48 | Quercetin arabinoside | 434 | 433 > 300 | 40 | 20 | Quercetin-3-O-Glucoside | 0.70 ± 0.01 | 0.62 ± 0.03 |
| 49 | Quercetin glucoside | 464 | 463 > 300 | 40 | 30 | Quercetin-3-O-Glucoside | 20.30 ± 0.76 | 4.51 ± 0.11 |
| 50 | Quercetin rhmanoside | 478 | 477 > 301 | 40 | 25 | Quercetin-3-O-Glucoside | 2.07 ± 0.07 | n.d. |
| 51 | Quercetin glucuronide | 478 | 477 > 301 | 40 | 25 | Quercetin-3-O-Glucoside | 67.8 ± 0.18 | 0.04 ± 0.00 |
| 52 | Quercetin acetylglucoside | 506 | 505 > 300 | 40 | 25 | Quercetin-3-O-Glucoside | 2.99 ± 0.09 | 0.02 ± 0.02 |
| 53 | Quercetin arabinoside glucoside | 596 | 595 > 300 | 40 | 30 | Quercetin-3-O-Glucoside | 374.40 ± 22.70 | 0.39 ± 0.08 |
| 54 | Quercetin rutinoside | 610 | 609 > 300 | 40 | 30 | Quercetin-3-O-rutinoside | 6.73 ± 0.19 | 0.23 ± 0.11 |
| 55 | Quercetin diglucoside | 626 | 625 > 300 | 40 | 30 | Quercetin-3-O-Glucoside | 294.00 ± 13.1 | 1.82 ± 0.17 |
| 56 | Quercetin glucoside rhamnoside | 756 | 755 > 300 | 40 | 35 | Quercetin-3-O-Glucoside | 4.98 ± 1.12 | n.d. |
| 57 | Isorhamnetin | 316 | 315 > 300 | 40 | 15 | Isorhamnetin | 0.01 ± 0.00 | n.d. |
| 58 | Isorhamnetin derivative | 316 | 300 > 315 | 40 | 15 | Isorhamnetin | 2.19 ± 0.09 | n.d. |
| 59 | Isorhamnetin arabinoside | 448 | 447 > 315 | 40 | 20 | Isorhamnetin | 0.04 ± 0.00 | 0.49 ± 0.13 |
| 60 | Isorhamnetin glucoside | 478 | 477 > 315 | 40 | 20 | Isorhamnetin | 0.07 ± 0.00 | 0.05 ± 0.02 |
| 61 | Isorhamnetin glucuronide | 492 | 491 > 315 | 40 | 20 | Isorhamnetin | 9.20 ± 0.06 | n.d. |
| 62 | Isorhamnetin arabinoside glucoside | 610 | 609 > 315 | 40 | 30 | Isorhamnetin | 0.11 ± 0.01 | n.d. |
| 63 | Kaempferol arabinoside | 418 | 417 > 284 | 40 | 20 | Kaempferol-3-O-Glucoside | 0.26 ± 0.01 | 0.10 ± 0.03 |
| 64 | Kaempferol glucoside | 448 | 447 > 284 | 40 | 20 | Kaempferol-3-O-Glucoside | 4.81 ± 0.18 | 0.65 ± 0.01 |
| 65 | Kaempferol rutinoside | 594 | 593 > 284 | 40 | 30 | Kaempferol-3-O-Glucoside | 13.2 ± 0.27 | n.d. |
| 66 | Kaempferol arabinoside glucoside | 580 | 579 > 284 | 40 | 30 | Kaempferol-3-O-Glucoside | 160.7 ± 2.46 | 0.25 ± 0.03 |
| 67 | Naringenin | 272 | 271 > 151 | 40 | 15 | Quercetin-3-O-Glucoside | 0.27 ± 0.09 | 0.20 ± 0.01 |
| 68 | Naringenin glucoside | 434 | 433 > 271 | 40 | 20 | Quercetin-3-O-Glucoside | 0.59 ± 0.00 | 0.86 ± 0.14 |
| 69 | Sakuratetin | 286 | 285 > 199 | 40 | 20 | Quercetin-3-O-Glucoside | 0.25 ± 0.01 | n.d. |
| 70 | Luteolin | 286 | 285 > 133 | 40 | 20 | Quercetin-3-O-Glucoside | n.d. | 0.06 ± 0.02 |
| 71 | Luteolin arabinoside glucoside | 580 | 579 > 285 | 40 | 30 | Quercetin-3-O-Glucoside | 6.07 ± 0.34 | n.d. |
| Terpenes | 2.82 ± 1.86 | 0.62 ± 0.13 | ||||||
| 72 | Penstemide | 444 | 443 > 119 | 40 | 25 | Quercetin-3-O-Glucoside | 2.82 ± 1.86 | 0.62 ± 0.13 |
| Total phenolic compounds | 6836.32 ± 64.66 | 1514.62 ± 578.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velderrain-Rodríguez, G.R.; Salvia-Trujillo, L.; Martín-Belloso, O. Lipid Digestibility and Polyphenols Bioaccessibility of Oil-in-Water Emulsions Containing Avocado Peel and Seed Extracts as Affected by the Presence of Low Methoxyl Pectin. Foods 2021, 10, 2193. https://doi.org/10.3390/foods10092193
Velderrain-Rodríguez GR, Salvia-Trujillo L, Martín-Belloso O. Lipid Digestibility and Polyphenols Bioaccessibility of Oil-in-Water Emulsions Containing Avocado Peel and Seed Extracts as Affected by the Presence of Low Methoxyl Pectin. Foods. 2021; 10(9):2193. https://doi.org/10.3390/foods10092193
Chicago/Turabian StyleVelderrain-Rodríguez, Gustavo R., Laura Salvia-Trujillo, and Olga Martín-Belloso. 2021. "Lipid Digestibility and Polyphenols Bioaccessibility of Oil-in-Water Emulsions Containing Avocado Peel and Seed Extracts as Affected by the Presence of Low Methoxyl Pectin" Foods 10, no. 9: 2193. https://doi.org/10.3390/foods10092193