Use of Micellar Delivery Systems to Enhance Curcumin’s Stability and Microbial Photoinactivation Capacity
Abstract
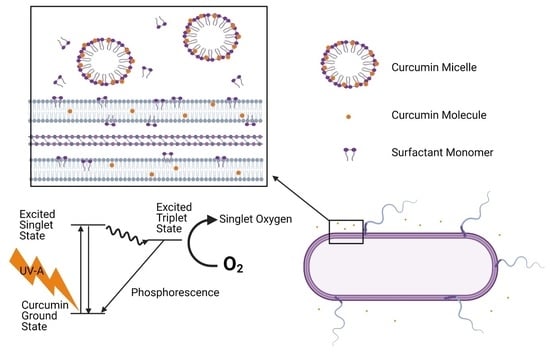
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Stock Curcumin-Surfactant Solutions
2.3. Maximum Curcumin Loading Capacity of Surfactant Solutions
2.4. Stability of Stock Curcumin in Surfactant Solutions during Storage
2.5. Surface Potential of the Curcumin Micelles in the Stock Solutions
2.6. Bacterial Culture Conditions
2.7. Bacterial Photoinactivation
2.8. Fluorescence Lifetime Imaging Microscopy (FLIM)
2.9. Data Acquisition and Analysis
3. Results and Discussion
3.1. Influence of pH on the Photoinactivation Capacity of Unencapsulated Curcumin
3.2. Influence of Surfactant Level on Curcumin Stability in the Stock Solutions
3.3. Influence of Surfactant Level on Microbial Photoinactivation
3.4. Combined Effects on Microbial Photoinactivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulchholz, A.L.; Davidson, G.R.; Marks, B.P.; Todd, E.C.; Ryser, E.T. Transfer of Escherichia coli O157:H7 from equipment surfaces to fresh-cut leafy greens during processing in a model pilot-plant production line with sanitizer-free water. J. Food Prot. 2012, 75, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, W. Guidelines for the Use of Chlorine Bleach as a Sanitizer in Food Processing Operations. Oklahoma Cooperative Extension Service. Division of Agricultural Sciences and Natural Resources. 2016. Available online: https://extension.okstate.edu/fact-sheets/guidelines-for-the-use-of-chlorine-bleach-as-a-sanitizer-in-food-processing-operations.html (accessed on 10 June 2021).
- Chen, X.; Hung, Y.-C. Effects of organic load, sanitizer pH and initial chlorine concentration of chlorine-based sanitizers on chlorine demand of fresh produce wash waters. Food Control 2017, 77, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.; Van Haute, S.; Zhou, B.; Hapeman, C.J.; Millner, P.D.; Wang, Q.; Luo, Y. Impacts and interactions of organic compounds with chlorine sanitizer in recirculated and reused produce processing water. PLoS ONE 2018, 13, e0208945. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.L.; Palumbo, M.S.; Farrar, J.A.; Farver, T.B.; Cliver, D.O. Industry practices and compliance with U.S. Food and Drug Administration Guidelines among California sprout firms. J. Food Prot. 2003, 66, 1253–1259. [Google Scholar] [CrossRef]
- Hua, Z.; Korany, A.M.; El-Shinawy, S.H.; Zhu, M.-J. Comparative evaluation of different sanitizers against Listeria monocytogenes biofilms on major food-contact surfaces. Front. Microbiol. 2019, 10, 2462. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Min, D.B.; Boff, J.M. Chemistry and reaction of singlet oxygen in foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouyet, B.; Chapelon, R. Photochemical mechanisms in photosensitization. J. Phys. Colloq. 1987, 48, 247–251. [Google Scholar] [CrossRef]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging trends in the photodynamic inactivation (P.D.I.) applied to the food decontamination. Food Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef]
- Hardwick, J.P. Cytochrome P450 Function and Pharmacological Roles in Inflammation and Cancer; Academic Press: London, UK, 2015. [Google Scholar]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef]
- Dysart, J.S.; Patterson, M.S. Characterization of photofrin photobleaching for singlet oxygen dose estimation during photodynamic therapy of MLL cells in vitro. Phys. Med. Biol. 2005, 50, 2597–2616. [Google Scholar] [CrossRef] [PubMed]
- Luksiene, Z.; Peciulyte, D.; Jurkoniene, S.; Puras, R. Inactivation of possible fungal food contaminants by photosensitization. Food Technol. Biotechnol. 2005, 43, 335–341. [Google Scholar]
- Buchovec, I.; Vaitonis, Z.; Luksiene, Z. Novel approach to control Salmonella enterica by modern biophotonic technology: Photosensitization. J. Appl. Microbiol. 2009, 106, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Karaffa, L.S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publishing: London, UK, 2013. [Google Scholar]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- De Oliveira, E.F.; Tikekar, R.; Nitin, N. Combination of aerosolized curcumin and UV-A light for the inactivation of bacteria on fresh produce surfaces. Food Res. Int. 2018, 114, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Duan, Y.; Wang, J.; Yang, X.; Du, H.; Xi, Y.; Zhai, G. Curcumin-loaded mixed micelles: Preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv. 2015, 22, 50–57. [Google Scholar] [CrossRef]
- Leung, M.H.; Colangelo, H.; Kee, T.W. Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir 2008, 24, 5672–5675. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Uluata, S.; McClements, D.J.; Decker, E.A. Riboflavin-induced oxidation in fish oil-in-water emulsions: Impact of particle size and optical transparency. Food Chem. 2016, 213, 457–461. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Bacteriological Analytical Manual (BAM); Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2017. Available online: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm20,694 (accessed on 8 June 2021).
- Colaruotolo, L.A.; Peters, E.; Corradini, M.G. Novel luminescent techniques in aid of food quality, product development, and food processing. Curr. Opin. Food Sci. 2021, 42, 148–156. [Google Scholar] [CrossRef]
- Miraglia, D.B.; Rodríguez, J.L.; Minardi, R.M.; Schulz, P.C. Critical micelle concentration and HLB of the sodium oleate–hexadecyltrimethylammonium bromide mixed system. J. Surfactants Deterg. 2011, 14, 401–408. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of food-grade nanoemulsions using low-energy preparation methods: A review of available methods. Comp. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- Gaysinsky, S. Physicochemical and Antimicrobial Properties of Antimicrobials Encapsulated in Surfactant-Based Nanoparticles. Master’s Thesis, University of Tennesse, Knoxville, TN, USA, 2004. [Google Scholar]
- Ma, P.; Zeng, Q.; Tai, K.; He, X.; Yao, Y.; Hong, X.; Yuan, F. Development of stable curcumin nanoemulsions: Effects of emulsifier type and surfactant-to-oil ratios. J. Food Sci. Technol. 2018, 55, 3485–3497. [Google Scholar] [CrossRef] [PubMed]
- Páhi, A.B.; Király, Z.; Puskás, S. Mass spectrometric characterization of the nonionic Gemini surfactant Surfynol 465 and a microcalorimetric study of its micelle formation in water. Colloids Surf. A 2009, 345, 13–17. [Google Scholar] [CrossRef]
- Sato, S.; Kishimoto, H. Behavior of nonionic surfactant, Surfynol 465, in aqueous media. In Surfactants in Solution; Mittal, K.L., Ed.; Springer: New York, NY, USA, 1989. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Influence of organic matter, cations and surfactants on the antimicrobial activity of Melaleuca alternifolia (tea tree) oil in vitro. J. Appl. Microbiol. 1999, 86, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Uzhinov, B.M.; Ivanov, V.L.; Melnikov, M.Y. Molecular rotors as luminescent sensors of local viscosity and viscous flow in solution and organized systems. Russ. Chem. Rev. 2011, 80, 1179–1190. [Google Scholar] [CrossRef]
- Le Maire, M.; Moeller, J.V.; Champeil, P. Binding of a nonionic detergent to membranes: Flip-flop rate and location on the bilayer. Biochemistry 1987, 26, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- De La Maza, A.; Parra, J.; Garcia, M.; Ribosa, I.; Leal, J.S. Permeability changes in the phospholipid bilayer caused by nonionic surfactants. J. Colloid Interface Sci. 1992, 148, 310–316. [Google Scholar] [CrossRef]
- Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Stability and antimicrobial efficiency of eugenol encapsulated in surfactant micelles as affected by temperature and pH. J. Food Prot. 2005, 68, 1359–1366. [Google Scholar] [CrossRef]
- Dahl, T.A.; Mcgowan, W.M.; Shand, M.A.; Srinivasan, V.S. Photokilling of bacteria by the natural dye curcumin. Arch. Microbiol. 1989, 151, 183–185. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157: H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
- Agel, M.R.; Baghdan, E.; Pinnapireddy, S.R.; Lehmann, J.; Schäfer, J.; Bakowsky, U. Curcumin loaded nanoparticles as efficient photoactive formulations against Gram-positive and Gram-negative bacteria. Colloids Surf. B 2019, 178, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Mozes, N. Microbial Cell Surface Analysis; VCH Publishers: New York, NY, USA, 1991. [Google Scholar]
- Maisch, T.; Szeimies, R.-M.; Jori, G.; Abels, C. Antibacterial photodynamic therapy in dermatology. Photochem. Photobiol. Sci. 2004, 3, 907–917. [Google Scholar] [CrossRef] [PubMed]









| Curcumin/Surfactant Solution | Mean Particle Diameter Z-AverZage (nm) ** | Polydispersity Index (PDI) ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Curcumin Conc. (µM) | Surfactant | 4 °C | 20 °C | 4 °C | 20 °C | |||||
| Type | Level | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | |
| 20 | None | - | - | - | - | - | - | - | - | - |
| S465 | Below CMC | 6.8 ± 2.1 Aa | 7.6 ± 2.2 Aa | 6.8 ± 2.1 Aa | 7.1 ± 1.8 Aa | 0.11 ± 0.06 Aa | 0.09 ± 0.08 Aa | 0.11 ± 0.06 Aa | 0.16 ± 0.06 Aa | |
| Near CMC | 5.8 ± 0.7 Aa | 5.3 ± 0.2 Aa | 5.8 ± 0.7 Aa | 7.1 ± 1.8 Aa | 0.11 ± 0.07 Aa | 0.06 ± 0.05 Aa | 0.11 ± 0.07 Aa | 0.08 ± 0.02 Aa | ||
| Above CMC | 6.6 ± 1.8 Aa | 6.2 ± 0.7 Aa | 6.6 ± 1.8 Aa | 8.3 ± 2.3 Aa | 0.13 ± 0.03 Aa | 0.18 ± 0.03 Aa | 0.13 ± 0.03 Aa | 0.14 ± 0.01 Aa | ||
| T80 | Below CMC * | - | - | - | - | - | - | - | - | |
| Near CMC | 15 ± 1.0 Ab | 16 ± 5.3 Ab | 15 ± 1.0 Ab | 17 ± 2.8 Ab | 0.21 ± 0.09 Aa | 0.26 ± 0.08 Aa | 0.21 ± 0.09 Aa | 0.24 ± 0.07 Ab | ||
| Above CMC | 15 ± 4.0 Ab | 19 ± 6.8 Ab | 15 ± 4.0 Ab | 17 ± 2.8 Ab | 0.29 ± 0.16 Aa | 0.33 ± 0.28 Aa | 0.29 ± 0.16 Aa | 0.23 ± 0.02 Ac | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, V.; Ruiz-Ramirez, S.; Chuesiang, P.; McLandsborough, L.A.; McClements, D.J.; Corradini, M.G. Use of Micellar Delivery Systems to Enhance Curcumin’s Stability and Microbial Photoinactivation Capacity. Foods 2021, 10, 1777. https://doi.org/10.3390/foods10081777
Ryu V, Ruiz-Ramirez S, Chuesiang P, McLandsborough LA, McClements DJ, Corradini MG. Use of Micellar Delivery Systems to Enhance Curcumin’s Stability and Microbial Photoinactivation Capacity. Foods. 2021; 10(8):1777. https://doi.org/10.3390/foods10081777
Chicago/Turabian StyleRyu, Victor, Silvette Ruiz-Ramirez, Piyanan Chuesiang, Lynne A. McLandsborough, David Julian McClements, and Maria G. Corradini. 2021. "Use of Micellar Delivery Systems to Enhance Curcumin’s Stability and Microbial Photoinactivation Capacity" Foods 10, no. 8: 1777. https://doi.org/10.3390/foods10081777