Physicochemical and Microstructural Characterization of Whey Protein Films Formed with Oxidized Ferulic/Tannic Acids
Abstract
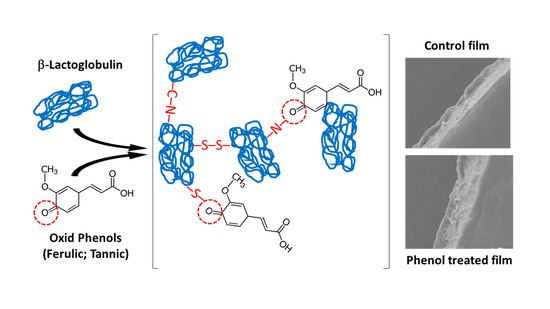
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Oxidation Phenolic Acids
2.3. Film-Forming Solutions Preparation and Film Casting
2.4. Measurement of Protein Changes
2.4.1. Total Sulfhydryl Content and Free Amines
2.4.2. Surface Hydrophobicity
2.4.3. ζ-Potential and Particle Size
2.4.4. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS–PAGE)
2.5. Assessment of Film Properties
2.5.1. Mechanical Properties
2.5.2. Swelling
2.5.3. Water Vapor Permeability (WVP)
2.5.4. Microstructure and Morphology
2.5.5. Light Transmittance and Transparency
2.5.6. Thermal Properties
2.5.7. Film Protein Leachability
2.5.8. Film Digestibility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Protein Modification and Physicochemical Changes of Film-Forming Solutions
3.2. Mechanical Properties
3.3. Swelling and WVP
3.4. Microstructure
3.5. Light Transmission and Transparency
3.6. Thermal Properties
3.7. Protein Leachability
3.8. Digestibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Fernandes, J.; da Silva, S.B.; Pintado, M.M.; Malcata, F. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Crit. Rev. Food Sci. Nutr. 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.L.; Rentfrow, G.K.; Newman, M.C. Oxidation promotes cross-linking but impairs film-forming properties of whey proteins. J. Food Eng. 2013, 115, 11–19. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Zhang, T.; Mu, G.; Jiang, S.; Zhu, X.; Tuo, Y.; Qian, F. Evaluation of the properties of whey protein films with modifications. J. Food Sci. 2021, 86, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Krochta, J.M. Edible protein films and coatings. In Food Proteins and Their Applications; Damodaran, S., Paraf, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 529–549. [Google Scholar]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Wihodo, M.; Moraru, C.I. Physical and chemical methods used to enhance the structure and mechanical properties of protein films: A review. J. Food Eng. 2013, 114, 292–302. [Google Scholar] [CrossRef]
- Buchert, J.; Ercili-Cura, D.; Ma, H.; Gasparetti, C.; Monogioudi, E.; Faccio, G.; Mattinen, M.; Boer, H.; Partanen, R.; Selinheimo, E.; et al. Crosslinking Food Proteins for Improved Functionality. Annu. Rev. Food Sci. Technol. 2010, 1, 113–138. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Mariotti, C.E.; Conti, B.; Boccaccini, A.R. Electrophoretic deposition of ferulic acid loaded bioactive glass/chitosan as antibacterial and bioactive composite coatings. Surf. Coat. Technol. 2021, 405, 126657. [Google Scholar] [CrossRef]
- Hazer, B.; Ashby, R.D. Synthesis of a novel tannic acid-functionalized polypropylene as antioxidant active-packaging materials. Food Chem. 2021, 344, 128644. [Google Scholar] [CrossRef]
- Cao, N.; Fu, Y.; He, J. Mechanical properties of gelatin films cross-linked, respectively, by ferulic acid and tannin acid. Food Hydrocoll. 2007, 21, 575–584. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Lewandowska, K.; Sionkowska, A. Modification of Collagen Properties with Ferulic Acid. Materials 2020, 13, 3419. [Google Scholar] [CrossRef]
- Santos, T.; Filho, M.D.S.M.S.; Muniz, C.R.; Morais, J.P.S.; Kotzebue, L.R.V.; Pereira, A.L.S.; Mc Azeredo, H. Zein films with unoxidized or oxidized tannic acid. J. Sci. Food Agric. 2017, 97, 4580–4587. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Xie, L.; Wehling, R.L.; Ciftci, O.; Zhang, Y. Formation of complexes between tannic acid with bovine serum albumin, egg ovalbumin and bovine beta-lactoglobulin. Food Res. Int. 2017, 102, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Balange, A.K.; Benjakul, S. Cross-linking activity of oxidised tannic acid towards mackerel muscle proteins as affected by protein types and setting temperatures. Food Chem. 2010, 120, 268–277. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.; Butterfield, D. Chemical, Physical, and Gel-forming Properties of Oxidized Myofibrils and Whey- and Soy-protein Isolates. J. Food Sci. 2000, 65, 811–818. [Google Scholar] [CrossRef]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of hydrophobic and net change to the solubility of milk and soy proteins. J. Food Sci. 1985, 50, 486–491. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- ASTM. Standard test method for tensile properties of thin plastic sheeting. Standard designation D882–00. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 2000; pp. 160–168. [Google Scholar]
- ASTM. Standard test methods for water vapor transmission of materials. Standard designation E96-E80. In Annual Book of ASTM Standards; ASTM: Philadelphia, PA, USA, 1989; pp. 730–739. [Google Scholar]
- Ustunol, Z.; Mert, B. Water Solubility, Mechanical, Barrier, and Thermal Properties of Cross-linked Whey Protein Isolate-based Films. J. Food Sci. 2006, 69, FEP129–FEP133. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, Y.L. Antioxidant and Bile Acid Binding Activity of Buckwheat Protein in Vitro Digests. J. Agric. Food Chem. 2009, 57, 4372–4380. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Myoprotein–phytophenol interaction: Implications for muscle food structure-forming properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2801–2824. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Free. Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Gibson, S.M. Plant phenolics as cross-linkers of gelatin gels and gelatin-based coacervates for use as food ingredients. Food Hydrocoll. 2004, 18, 81–89. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical Modification of Gelatin by a Natural Phenolic Cross-linker, Tannic Acid. J. Agric. Food Chem. 2010, 58, 6809–6815. [Google Scholar] [CrossRef] [PubMed]
- Stammers, M.; Niewczas, I.S.; Segonds-Pichon, A.; Clark, J. Mechanical stretching changes crosslinking and glycation levels in the collagen of mouse tail tendon. J. Biol. Chem. 2020, 295, 10572–10580. [Google Scholar] [CrossRef]
- Keppler, J.K.; Schwarz, K.; van der Goot, A.J. Covalent modification of food proteins by plant-based ingredients (polyphenols and organosulphur compounds): A commonplace reaction with novel utilization potential. Trends Food Sci. Technol. 2020, 101, 38–49. [Google Scholar] [CrossRef]
- Xue, F.; Zhao, M.; Liu, X.; Chu, R.; Qiao, Z.; Li, C.; Adhikari, B. Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols. Futur. Foods 2021, 3, 100033. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Zhang, C.; Chai, W.S.; Chew, K.W.; Du, A.; Show, P.L. Characterization of whey protein isolate and pectin composite film catalyzed by small laccase from Streptomyces coelicolor. Environ. Technol. Innov. 2020, 19, 100999. [Google Scholar] [CrossRef]
- Tonyali, B.; Cikrikci, S.; Oztop, M.H. Physicochemical and microstructural characterization of gum tragacanth added whey protein based films. Food Res. Int. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Roozalipour, S.P.L.; Berton-Carabin, C.C.; Nikiforidis, C.V.; van der Linden, E.; Sagis, L.M. Air-water interfacial and foaming properties of whey protein sinapic acid mixtures. Food Hydrocoll. 2021, 112, 106467. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Lenart, A.; Voilley, A. Water vapour permeability, thermal and wetting properties of whey protein isolate based edible films. Int. Dairy J. 2010, 20, 53–60. [Google Scholar] [CrossRef]
- Yilmaz, K.; Turhan, S.; Saricaoglu, F.T.; Tural, S. Improvement of physicochemical, mechanical, thermal and surface properties of anchovy by-product protein films by addition of transglutaminase, and the correlation between secondary structure and mechanical properties. Food Packag. Shelf Life 2020, 24, 100483. [Google Scholar] [CrossRef]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int. J. Biol. Macromol. 2009, 44, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Ustunol, Z. Thermal Properties, Heat Sealability and Seal Attributes of Whey Protein Isolate/Lipid Emulsion Edible Films. J. Food Sci. 2001, 66, 985–990. [Google Scholar] [CrossRef]
- Jafari, S.M.; Khanzadi, M.; Mirzaei, H.; Dehnad, D.; Chegini, F.K.; Maghsoudlou, Y. Hydrophobicity, thermal and micro-structural properties of whey protein concentrate–pullulan–beeswax films. Int. J. Biol. Macromol. 2015, 80, 506–511. [Google Scholar] [CrossRef]
- Kadam, D.M.; Thunga, M.; Wang, S.; Kessler, M.; Grewell, D.; Lamsal, B.; Yu, C. Preparation and characterization of whey protein isolate films reinforced with porous silica coated titania nanoparticles. J. Food Eng. 2013, 117, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Janjarasskul, T.; Tananuwong, K.; Phupoksakul, T.; Thaiphanit, S. Fast dissolving, hermetically sealable, edible whey protein isolate-based films for instant food and/or dry ingredient pouches. LWT 2020, 134, 110102. [Google Scholar] [CrossRef]
- Kananen, A.; Savolainen, J.; Mäkinen, J.; Perttilä, U.; Myllykoski, L.; Pihlanto-Leppälä, A. Influence of chemical modification of whey protein conformation on hydrolysis with pepsin and trypsin. Int. Dairy J. 2000, 10, 691–697. [Google Scholar] [CrossRef]
- Peña-Ramos, E.; Xiong, Y. Antioxidative Activity of Whey Protein Hydrolysates in a Liposomal System. J. Dairy Sci. 2001, 84, 2577–2583. [Google Scholar] [CrossRef]
- Ma, R.; Lin, Z.; Wu, Y.; Gao, Z.; Hu, B.; Xu, L.; Fang, Y.; Nishinari, K. Modulating the in vitro gastric digestion of heat-induced beta-lactoglobulin aggregates: Incorporation with polysaccharide. Food Chem. 2021, 354, 129506. [Google Scholar] [CrossRef]
- Otte, J.; Lomholt, S.B.; Halkier, T.; Qvist, K.B. Identification of Peptides in Aggregates Formed during Hydrolysis of β-Lactoglobulin B with a Glu and Asp Specific Microbial Protease. J. Agric. Food Chem. 2000, 48, 2443–2447. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Control | OFA (w/w) | OTA (%, w/w) | ||
|---|---|---|---|---|---|
| 2.5% | 5.0% | 2.5% | 5.0% | ||
| SH (nmol/mg protein) | 21.8 ± 0.3 a | 16.4 ± 0.5 b | 11.4 ± 0.2 c | 4.2 ± 0.2 d | 1.2 ± 1.5 e |
| NH2 (μmol/mg protein) | 0.79 ± 0.02 a | 0.75 ± 0.02 b | 0.72 ± 0.01 b | 0.62 ± 0.01 c | 0.59 ± 0.01 d |
| Hydrophobicity | 2.59 ± 0.16 a | 2.62 ± 0.07 a | 2.48 ± 0.07 a | 1.94 ± 0.02 b | 1.72 ± 0.05 c |
| Particle size (nm) | 85 ± 9 a,b | 78 ± 1 b | 83 ± 6 b | 117 ± 18 a | 112 ± 48 a |
| ζ-potential (mV) | −26.6 ± 1.9 a | −26.5 ± 2.2 a | −25.5 ± 1.8 a | −24.8 ± 1.7 a | −25.6 ± 2.4 a |
| Property | Control | OFA (w/w) | OTA (%, w/w) | ||
|---|---|---|---|---|---|
| 2.5% | 5.0% | 2.5% | 5.0% | ||
| TS (MPa) | 4.5 ± 0.2 c | 4.9 ± 0.7 b,c | 5.2 ± 0.5 b | 6.7 ± 0.3 a | 6.7 ± 0.40 a |
| EAB (%) | 24.2 ± 4.1 b | 34.9 ± 10.0 a | 35.5 ± 11.9 a | 20.5 ± 4.3 b | 15.2 ± 1.8 b |
| Young’s modulus (MPa) | 190 ± 11 c | 215 ± 22 b,c | 238 ± 26 b | 358 ± 19 a | 376 ± 4 a |
| Swelling | 139 ± 61 b | 171 ± 48 b | 193 ± 7 b | 325 ± 21 a | 318 ± 86 a |
| WVP (g·mm/kPa·h·m2) | 1.02 ± 0.21 a | 1.00 ± 0.01 a | 0.94 ± 0.01 a | 0.95 ± 0.04 a | 0.95 ± 0.11 a |
| Treatment | Light Transmittance (%) at Different Wavelengths (nm) | Transparency | ||||||
|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 800 | ||
| 0% | 0 | 0 | 70.7 ± 2.0 a | 78.5 ± 1.8 a | 82.9 ± 1.7 a | 85.0 ± 1.6 a | 89.0 ± 1.6 a | 1.0 ± 0.1 c |
| 2.5% OFA | 0 | 0 | 12.7 ± 0.5 b | 67.1 ± 3.7 b | 80.2 ± 4.4 a | 83.0 ± 4.2 a | 87.7 ± 3.7 a | 1.1 ± 0.3 c |
| 5.0% OFA | 0 | 0 | 0.4 ± 0.3 e | 44.9 ± 5.1 c | 80.5 ± 0.4 a | 84.1 ± 0.5 a | 88.8 ± 0.3 a | 1.1 ± 0.0 c |
| 2.5% OTA | 0 | 0 | 8.9 ± 0.9 c | 29.7 ± 1.8 d | 58.4 ± 2.3 b | 69.2 ± 2.6 b | 77.5 ± 2.6 b | 2.7 ± 0.3 b |
| 5.0% OTA | 0 | 0 | 2.4 ± 0.7 d | 14.7 ± 2.8 e | 45.0 ± 4.7 c | 59.3 ± 4.9 c | 69.9 ± 4.1 c | 4.5 ± 0.7 a |
| Digestion | Treatment | Hydrolysis Time (min) | ||||||
|---|---|---|---|---|---|---|---|---|
| Pepsin | Pancreatin | |||||||
| 0 2 | 30 | 60 | 90 | 120 | 180 | 240 | ||
| 4% Pepsin (1 h 37 °C, pH 2.0) | 0% | (35.2 a) | 56.0 ± 1.4 a | 44.8 ± 0.6 a,b | 56.0 ± 6.2 a | 72.7 ± 3.7 b | 70.0 ± 2.6 c | 85.8 ± 0.6 b |
| 2.5% OFA | (32.3 a,b) | 47.1 ± 0.3 b | 44.0 ± 0.3 a,b | 48.0 ± 2.8 b | 62.2 ± 0.9 c | 81.1 ± 0.3 b | 91.0 ± 4.6 a | |
| 5.0% OFA | (29.0 b) | 44.1 ± 0.3 c | 46.6 ± 3.1 a | 40.7 ± 2.1 c | 57.9 ± 0.7 c,d | 80.0 ± 2.1 b | 82.4 ± 2.8 b | |
| ↓ | ||||||||
| 4% Pancreatin (4 h, 37 °C, pH 7.5) | 2.5% OTA | (27.6 b) | 49.3 ± 1.4 a,b | 42.4 ± 0.4 b | 54.7 ± 1.9 a | 77.2 ± 2.2 a | 89.0 ± 2.2 a | 89.1 ± 0.4 a |
| 5.0% OTA | (28.5 b) | 40.7 ± 0.4 d | 28.4 ± 0.4 c | 34.4 ± 1.4 d | 60.0 ± 2.1 c | 62.1 ± 2.8 d | 65.9 ± 0.4 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiong, Y.L. Physicochemical and Microstructural Characterization of Whey Protein Films Formed with Oxidized Ferulic/Tannic Acids. Foods 2021, 10, 1599. https://doi.org/10.3390/foods10071599
Wang Y, Xiong YL. Physicochemical and Microstructural Characterization of Whey Protein Films Formed with Oxidized Ferulic/Tannic Acids. Foods. 2021; 10(7):1599. https://doi.org/10.3390/foods10071599
Chicago/Turabian StyleWang, Yaosong, and Youling L. Xiong. 2021. "Physicochemical and Microstructural Characterization of Whey Protein Films Formed with Oxidized Ferulic/Tannic Acids" Foods 10, no. 7: 1599. https://doi.org/10.3390/foods10071599