Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies
Abstract
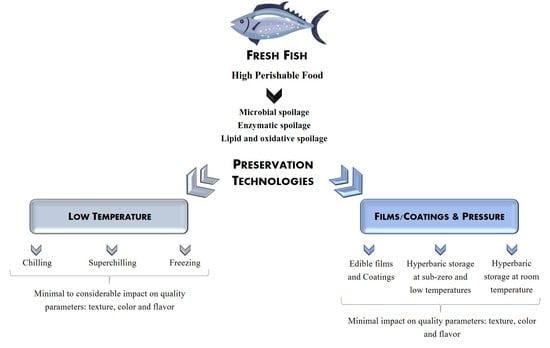
:1. Introduction
1.1. Fish Spoilage
1.1.1. Autolytic Enzymatic Spoilage
1.1.2. Lipid Hydrolysis and Oxidative Spoilage
1.1.3. Microbial Spoilage
2. Chilling, Superchilling, and Freezing
2.1. Chilling
2.2. Superchilling
2.3. Freezing
3. Emergent Preservation Techniques
3.1. Edible Films and Coatings
3.2. Hyperbaric Storage: A Novel Methodology for Fish Preservation
3.2.1. Hyperbaric Storage
3.2.2. Hyperbaric Storage at Subzero and Low Temperatures
3.2.3. Hyperbaric Storage at Room Temperatures
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A Comprehensive Review on Freshness of Fish and Assessment: Analytical Methods and Recent Innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Ježek, F.; Buchtová, H. Physical and Chemical Changes in Fresh Chilled Muscle Tissue of Common Carp (Cyprinus Carpio L.) Packed in a Modified Atmosphere. Acta Vet. Brno 2007, 76, S83–S92. [Google Scholar] [CrossRef] [Green Version]
- Ghaly, A.; Dave, D.; Budge, S.; Brooks, M. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A. Nutritional aspects of fish. In Producer to Consumer, Integrated Approach to Quality; Luten, J., Börrensem, T., Oehlenschläger, J., Eds.; Elsevier Science: London, UK, 1997; pp. 587–607. [Google Scholar]
- Simpson, B.K.; Nollet, L.M.L.; Toldrá, F.; Benjakul, S.; Paliyath, G.; Hui, Y.H. Food Biochemistry and Food Processing, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 13–288. ISBN 0813803780. [Google Scholar]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination Using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Kolakowska, A.; Olley, J.; Dunstan, G.A. Fish lipids. In Chemical and Functional Properties of Food Lipids; Zdzislaw, Z., Sikorski, E., Kolakowska, A., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 2010; pp. 221–265. [Google Scholar]
- Gram, L.; Huss, H.H. Microbiological Spoilage of Fish and Fish Products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Boziaris, I.S. Introduction to seafood processing-assuring quality and safety of seafood. In Seafood Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 1–8. [Google Scholar]
- FAO. Post-Harvest Changes in Fish. FAO—Fisheries and Aquaculture Department, Food and Agriculture Organization; FAO: Rome, Italy, 2005. [Google Scholar]
- Stagg, N.J.; Amato, P.M.; Giesbrecht, F.; Lanier, T.C. Autolytic Degradation of Skipjack Tuna during Heating as Affected by Initial Quality and Processing Conditions. J. Food Sci. 2012, 77, C149–C155. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zhu, J.; Wang, Y.; Fu, L.; Xuan, W. Postmortem Changes in Yellow Grouper (Epinephelus Awoara) Fillets Stored under Vacuum Packaging at 0 °C. Food Chem. 2011, 126, 896–901. [Google Scholar] [CrossRef]
- Kostaki, M.; Giatrakou, V.; Savvaidis, I.N.; Kontominas, M.G. Combined Effect of MAP and Thyme Essential Oil on the Microbiological, Chemical and Sensory Attributes of Organically Aquacultured Sea Bass (Dicentrarchus Labrax) Fillets. Food Microbiol. 2009, 26, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of Thyme Essential Oil and Packaging Treatments on Fresh Mediterranean Swordfish Fillets during Storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Genç, I.Y.; Esteves, E.; Aníbal, J.; Diler, A. Effects of Chilled Storage on Quality of Vacuum Packed Meagre Fillets. J. Food Eng. 2013, 115, 486–494. [Google Scholar] [CrossRef]
- Hultin, H.O. Oxidation of lipids in seafoods. In Seafoods Chemistry, Processing Technology and Quality; Shahidi, F., Botta, J.R., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 49–74. [Google Scholar]
- Huis In’t Veld, J.H.J. Microbial and Biochemical Spoilage of Foods: An Overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Erickson, M. Lipid oxidation: Flavor and nutritional quality deterioration in frozen foods. In Quality in Frozen Food; Erickson, M., Hung, Y.-C., Eds.; Chapman and Hall: New York, NY, USA, 1997; pp. 141–173. [Google Scholar]
- Khayat, A.; Schwall, D. Lipid Oxidation in Seafood. Food Technol. 1983, 37, 130–140. [Google Scholar]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A Review of Analytical Methods Measuring Lipid Oxidation Status in Foods: A Challenging Task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Comi, G. Chapter 8—Spoilage of meat and fish. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Bender, D.A., Purchase, R., Ruthven, A.D., Waldron, K.W., Johnson, I.T., Fenwick, G.R., Kress-Rogers, E., Bergenstahl, B., Monnier, V., Baynes, J., et al., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 179–210. ISBN 978-0-08-100502-6. [Google Scholar]
- Sperber, W.H.; Doyle, M.P. (Eds.) Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: Griffith, GA, USA, 2010; ISBN 978-1-4419-0826-1. [Google Scholar]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.K.S. Biogenic Amines in Seafood: A Review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Chopin, C.; Cornet, J.; Smith-Ravin, J.; Rochefort, K.; Leroi, F. Effect of Vacuum and Modified Atmosphere Packaging on the Microbiological, Chemical and Sensory Properties of Tropical Red Drum (Sciaenops Ocellatus) Fillets Stored at 4 °C. Int. J. Food Microbiol. 2018, 266, 31–41. [Google Scholar] [CrossRef]
- Church, N. MAP Fish and Crustaceans Sensory Enhancement. Food Sci. Technol. Today 1998, 12, 73–83. [Google Scholar]
- Gram, L.; Huss, H.H. Fresh and processed fish and shellfish. In The Microbiological Safety and Quality of Foods; Lund, B.M., Baird-Parker, A.C., Gould, G.W., Eds.; Chapman and Hall: London, UK, 2000; pp. 472–506. [Google Scholar]
- Kim, S.-H.; Ben-Gigirey, B.; Barros-Velázquez, J.; Price, R.J.; An, H. Histamine and Biogenic Amine Production by Morganella Morganii Isolated from Temperature-Abused Albacore. J. Food Prot. 2000, 63, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Tahmouzi, S.; Ghasemlou, M.; Aliabadi, F.S.; Shahraz, F.; Hosseini, H.; Khaksar, R. Histamine Formation and Bacteriological Quality in Skipjack Tuna (Katsuwonus Pelamis): Effect of Defrosting Temperature. J. Food Process. Preserv. 2013, 37, 306–313. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid Poisoning: A Review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Leisner, J.J.; Gram, L. Fish: Spoilage of Fish. In Encyclopedia of Food Microbiology; Academic press: Cambridge, MA, USA, 2014; pp. 932–937. [Google Scholar]
- Shawyer, M.; Pizzali, M. (Eds.) The use of ice on small fishing vessels. FAO Fisheries Technical Paper 436. In Food and Agriculture Organization of the United Nation; FAO: Rome, Italy, 2003; pp. 1–34. ISBN 92-5-105010-4. [Google Scholar]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Kolsaker, K. Superchilling of Food: A Review. J. Food Eng. 2011, 107, 141–146. [Google Scholar] [CrossRef]
- Brackett, R.E. Microbiological Safety of Chilled Foods: Current Issues. Trends Food Sci. Technol. 1992, 3, 81–85. [Google Scholar] [CrossRef]
- Berk, Z. (Ed.) Chapter 19—Refrigeration—Chilling and freezing. In Food Process Engineering and Technology, 3rd ed.; Food Science and Technology; Academic Press: Cambridge, MA, USA, 2018; pp. 439–461. ISBN 978-0-12-812018-7. [Google Scholar]
- Graham, J.; Johnston, W.; Nicholson, F. Ice in Fisheries: Food and Agriculture Organization; FAO: Rome, Italy, 1993. [Google Scholar]
- Banerjee, R.; Maheswarappa, N.B. Superchilling of Muscle Foods: Potential Alternative for Chilling and Freezing. Crit. Rev. Food Sci. Nutr. 2019, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, O.M.; Haugland, A.; Hemmingsen, A.K.T.; Johansen, S.; Nordtvedt, T.S. Advances in Superchilling of Food—Process Characteristics and Product Quality. Trends Food Sci. Technol. 2008, 19, 418–424. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality Changes during Superchilled Storage of Cod (Gadus Morhua) Fillets. Food Chem. 2007, 105, 1067–1075. [Google Scholar] [CrossRef]
- Erikson, U.; Misimi, E.; Gallart-Jornet, L. Superchilling of Rested Atlantic Salmon: Different Chilling Strategies and Effects on Fish and Fillet Quality. Food Chem. 2011, 127, 1427–1437. [Google Scholar] [CrossRef]
- Eliasson, S.; Arason, S.; Margeirsson, B.; Bergsson, A.B.; Palsson, O.P. The Effects of Superchilling on Shelf-Life and Quality Indicators of Whole Atlantic Cod and Fillets. LWT 2019, 100, 426–434. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent Advances in Quality Retention of Non-Frozen Fish and Fishery Products: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Hoang, H.M.; Brown, T.; Indergard, E.; Leducq, D.; Alvarez, G. Life Cycle Assessment of Salmon Cold Chains: Comparison between Chilling and Superchilling Technologies. J. Clean. Prod. 2016, 126, 363–372. [Google Scholar] [CrossRef]
- Wang, T.; Sveinsdóttir, K.; Magnússon, H.; Martinsdóttir, E. Combined Application of Modified Atmosphere Packaging and Superchilled Storage to Extend the Shelf Life of Fresh Cod (Gadus Morhua) Loins. J. Food Sci. 2008, 73, S11–S19. [Google Scholar] [CrossRef]
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-Life Extension on Fillets of Atlantic Salmon (Salmo Salar) Using Natural Additives, Superchilling and Modified Atmosphere Packaging. Food Control 2009, 20, 1036–1042. [Google Scholar] [CrossRef]
- Gallart-Jornet, L.; Rustad, T.; Barat, J.M.; Fito, P.; Escriche, I. Effect of Superchilled Storage on the Freshness and Salting Behaviour of Atlantic Salmon (Salmo Salar) Fillets. Food Chem. 2007, 103, 1268–1281. [Google Scholar] [CrossRef]
- Duun, A.S.; Rustad, T. Quality of Superchilled Vacuum Packed Atlantic Salmon (Salmo Salar) Fillets Stored at −1.4 and −3.6 °C. Food Chem. 2008, 106, 122–131. [Google Scholar] [CrossRef]
- Stevik, A.M.; Duun, A.S.; Rustad, T.; O’Farrell, M.; Schulerud, H.; Ottestad, S. Ice Fraction Assessment by Near-Infrared Spectroscopy Enhancing Automated Superchilling Process Lines. J. Food Eng. 2010, 100, 169–177. [Google Scholar] [CrossRef]
- Kaale, L.D.; Eikevik, T.M.; Rustad, T.; Nordtvedt, T.S. Changes in Water Holding Capacity and Drip Loss of Atlantic Salmon (Salmo Salar) Muscle during Superchilled Storage. LWT Food Sci. Technol. 2014, 55, 528–535. [Google Scholar] [CrossRef] [Green Version]
- Olafsdottir, G.; Lauzon, H.L.; Martinsdóttir, E.; Oehlenschläger, J.; Kristbergsson, K. Evaluation of Shelf Life of Superchilled Cod (Gadus Morhua) Fillets and the Influence of Temperature Fluctuations during Storage on Microbial and Chemical Quality Indicators. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Lauzon, H.L.; Magnússon, H.; Sveinsdóttir, K.; Gudjónsdóttir, M.; Martinsdóttir, E. Effect of Brining, Modified Atmosphere Packaging, and Superchilling on the Shelf Life of Cod (Gadus Morhua) Loins. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Cyprian, O.; Lauzon, H.L.; Jóhannsson, R.; Sveinsdóttir, K.; Arason, S.; Martinsdóttir, E. Shelf Life of Air and Modified Atmosphere-packaged Fresh Tilapia (Oreochromis Niloticus) Fillets Stored under Chilled and Superchilled Conditions. Food Sci. Nutr. 2013, 1, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Gökoğlu, N.; Yerlikaya, P. Freezing and frozen storage of fish. In Seafood Chilling, Refrigeration and Freezing; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 186–207. [Google Scholar]
- Hall, G.M. Freezing and chilling of fish and fish products. In Fish Processing: Sustainability and New Opportunities; Wiley-Blackwell: Oxford, UK, 2010; pp. 77–97. ISBN 9781405190473. [Google Scholar]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. Effect of Low and Ultra-Low Temperature Applications during Freezing and Frozen Storage on Quality Parameters for Fish. Int. J. Refrig. 2016, 63, 37–47. [Google Scholar] [CrossRef]
- Burgaard, M.G.; Jørgensen, B.M. Effect of Frozen Storage Temperature on Quality-Related Changes in Rainbow Trout (Oncorhynchus Mykiss). J. Aquat. Food Prod. Technol. 2011, 20, 53–63. [Google Scholar] [CrossRef]
- Johnston, W.A.; Nicholson, F.J.; Roger, A. Freezing and Refrigerated Storage in Fisheries; Johnston, W.A., Ed.; FAO Fisheries Technical Paper-T340; Food & Agriculture Organization: Rome, Italy, 1994. [Google Scholar]
- Jessen, F.; Nielsen, J.; Larsen, E. Chilling and freezing of fish. In Seafood Processing; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 33–59. [Google Scholar]
- Muthukumarappan, K.; Marella, C.; Sunkesula, V. Food freezing technology. In Handbook of Farm, Dairy and Food Machinery Engineering; Academic press: Cambridge, MA, USA, 2019; pp. 389–415. [Google Scholar]
- Tsironi, T.; Houhoula, D.; Taoukis, P. Hurdle Technology for Fish Preservation. Aquac. Fish. 2020, 5, 65–71. [Google Scholar] [CrossRef]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M.C. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and Physical Characteristics of Edible Films, Based on κ- and ι-Carrageenans with the Addition of Lapacho Tea Extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Taoukis, P.S. Current Practice and Innovations in Fish Packaging. J. Aquat. Food Prod. Technol. 2018, 27, 1024–1047. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and Active Films and Coatings as Carriers of Natural Antioxidants for Lipid Food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Lee, K.T. Quality and Safety Aspects of Meat Products as Affected by Various Physical Manipulations of Packaging Materials. Meat Sci. 2010, 86, 138–150. [Google Scholar] [CrossRef]
- Alishahi, A.; Aïder, M. Applications of Chitosan in the Seafood Industry and Aquaculture: A Review. Food Bioprocess Technol. 2012, 5, 817–830. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of Chitosan Coatings Enriched with Cinnamon Oil on the Quality of Refrigerated Rainbow Trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Bahram, S.; Rezaie, M.; Soltani, M.; Kamali, A.; Abdollahi, M.; Khezri Ahmadabad, M.; Nemati, M. Effect of Whey Protein Concentrate Coating Cinamon Oil on Quality and Shelf Life of Refrigerated Beluga Sturegeon (Huso Huso). J. Food Qual. 2016, 39, 743–749. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Suthiluk, P.; Rawdkuen, S. Shelf Life Extension for Bluefin Tuna Slices (Thunnus Thynnus) Wrapped with Myofibrillar Protein Film Incorporated with Catechin-Kradon Extract. Food Control 2017, 79, 333–343. [Google Scholar] [CrossRef]
- Volpe, M.G.; Siano, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active Edible Coating Effectiveness in Shelf-Life Enhancement of Trout (Oncorhynchusmykiss) Fillets. LWT Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, Q.; Xu, Y.; Xia, W. The Shelf Life Extension of Refrigerated Grass Carp (Ctenopharyngodon Idellus) Fillets by Chitosan Coating Combined with Glycerol Monolaurate. Int. J. Biol. Macromol. 2017, 101, 448–454. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdia, F.T.; Mortazavia, S.A.; Koocheki, A.; Khazaei, N. Effect of Quince Seed Mucilage Edible Films Incorporated with Oregano or Thyme Essential Oil on Shelf Life Extension of Refrigerated Rainbow Trout Fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef]
- Kazemi, S.M.; Rezaei, M. Antimicrobial Effectiveness of Gelatin-Alginate Film Containing Oregano Essential Oil for Fish Preservation. J. Food Saf. 2015, 35, 482–490. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W.; Li, X. Quality Enhancement in Refrigerated Red Drum (Sciaenops Ocellatus) Fillets Using Chitosan Coatings Containing Natural Preservatives. Food Chem. 2013, 138, 821–826. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López De Lacey, A.; Gómez-Guillén, M.C.; López-Caballero, M.E.; Montero, P. Antimicrobial Activity of Composite Edible Films Based on Fish Gelatin and Chitosan Incorporated with Clove Essential Oil. J. Aquat. Food Prod. Technol. 2009, 18, 46–52. [Google Scholar] [CrossRef]
- Yordanov, D.G.; Angelova, G.V. High Pressure Processing for Foods Preserving. Biotechnol. Biotechnol. Equip. 2010, 24, 1940–1945. [Google Scholar] [CrossRef] [Green Version]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure-Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Saraiva, J.A.; Aubourg, S.P.; Vázquez, M.; Torres, J.A. Effect of High-Pressure Pre-Treatments on Enzymatic Activities of Atlantic Mackerel (Scomber Scombrus) during Frozen Storage. Innov. Food Sci. Emerg. Technol. 2014, 23, 18–24. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Y.; Zhang, H.; Qi, X.; Pan, D. Effects of High Hydrostatic Pressure Treatment on the Qualities of Cultured Large Yellow Croaker (Pseudosciaena Crocea) during Cold Storage. J. Food Process. Preserv. 2014. [Google Scholar] [CrossRef]
- Moreno, H.M.; Bargiela, V.; Tovar, C.A.; Cando, D.; Borderias, A.J.; Herranz, B. High Pressure Applied to Frozen Flying Fish (Parexocoetus Brachyterus) Surimi: Effect on Physicochemical and Rheological Properties of Gels. Food Hydrocoll. 2015, 48, 127–134. [Google Scholar] [CrossRef]
- Tironi, V.; de Lamballerie, M.; Le-Bail, A. Quality Changes during the Frozen Storage of Sea Bass (Dicentrarchus Labrax) Muscle after Pressure Shift Freezing and Pressure Assisted Thawing. Innov. Food Sci. Emerg. Technol. 2010, 11, 565–573. [Google Scholar] [CrossRef]
- Nakazawa, N.; Okazaki, E. Recent Research on Factors Influencing the Quality of Frozen Seafood. Fish Sci. 2020, 86, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Jannasch, H.W.; Eimhjellen, K.; Wirsen, C.O.; Farmanfarmaian, A. Microbial Degradation of Organic Matter in the Deep Sea. Science 1971, 171, 672–675. [Google Scholar] [CrossRef]
- Charm, S.E.; Longmaid, H.E., III; Carver, J. A Simple System for Extending Refrigerated, Nonfrozen Preservation of Biological Material Using Pressure. Cryobiology 1977, 14, 625–636. [Google Scholar] [CrossRef]
- Wagner, W.; Saul, A.; Pruss, A. International Equations for the Pressure along the Melting and along the Sublimation Curve of Ordinary Water Substance. J. Phys. Chem. Ref. Data 1994, 23, 515. [Google Scholar] [CrossRef] [Green Version]
- Kalichevsky, M.T.; Knorr, D.; Lillford, P.J. Potential Food Applications of High-Pressure Effects on Ice-Water Transitions. Trends Food Sci. Technol. 1995, 6, 253–259. [Google Scholar] [CrossRef]
- Ooide, A.; Kameyama, Y.; Iwata, N.; Uchio, R.; Karino, S.; Kanyama, N. Non-freezing preservation of fresh foods under subzero temperature. In High Pressure Bioscience; Hayashi, R.S.K., Shimada, S., Suzuki, A., Eds.; San-Ei Shuppan Co.: Kyoto, Japan, 1994; pp. 344–351. [Google Scholar]
- Ko, W.-C.; Hsu, K.-C. Changes in K Value and Microorganisms of Tilapia Fillet during Storage at High-Pressure, Normal Temperature. J. Food Prot. 2001, 64, 94–98. [Google Scholar] [CrossRef]
- Otero, L.; Pérez-Mateos, M.; López-Caballero, M.E. Hyperbaric Cold Storage versus Conventional Refrigeration for Extending the Shelf-Life of Hake Loins. Innov. Food Sci. Emerg. Technol. 2017, 41, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, L.G.; Lemos, Á.T.; Delgadillo, I.; Saraiva, J.A. Microbial and Physicochemical Evolution during Hyperbaric Storage at Room Temperature of Fresh Atlantic Salmon (Salmo Salar). Innov. Food Sci. Emerg. Technol. 2018, 45, 264–272. [Google Scholar] [CrossRef]
- Otero, L.; Pérez-Mateos, M.; Holgado, F.; Márquez-Ruiz, G.; López-Caballero, M.E. Hyperbaric Cold Storage: Pressure as an Effective Tool for Extending the Shelf-Life of Refrigerated Mackerel (Scomber Scombrus, L.). Innov. Food Sci. Emerg. Technol. 2019, 51, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Fidalgo, L.G.; Castro, R.; Trigo, M.; Aubourg, S.P.; Delgadillo, I.; Saraiva, J.A. Quality of Fresh Atlantic Salmon (Salmo Salar) under Hyperbaric Storage at Low Temperature by Evaluation of Microbial and Physicochemical Quality Indicators. Food Bioprocess Technol. 2019, 12, 1895–1906. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Autolytic Changes Involving Proteolytic Enzymes on Atlantic Salmon (Salmo Salar) Preserved by Hyperbaric Storage. LWT 2020, 118, 108755. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Simões, M.M.Q.; Casal, S.; Lopes-da-Silva, J.A.; Carta, A.M.S.; Delgadillo, I.; Saraiva, J.A. Physicochemical Parameters, Lipids Stability, and Volatiles Profile of Vacuum-Packaged Fresh Atlantic Salmon (Salmo Salar) Loins Preserved by Hyperbaric Storage at 10 °C. Food Res. Int. 2020, 127, 108740. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, L.G.; Simões, M.M.Q.; Casal, S.; Lopes-da-Silva, J.A.; Delgadillo, I.; Saraiva, J.A. Enhanced Preservation of Vacuum-Packaged Atlantic Salmon by Hyperbaric Storage at Room Temperature versus Refrigeration. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, L.G.; Pint Fernandeso, C.A.; Delgadillo, I.; Saraiva, J.A. Hyperbaric Storage of Vacuum-Packaged Fresh Atlantic Salmon (Salmo Salar) Loins by Evaluation of Spoilage Microbiota and Inoculated Surrogate-Pathogenic Microorganisms. Food Eng. Rev. 2021. [Google Scholar] [CrossRef]
- Bermejo-Prada, A.; Colmant, A.; Otero, L.; Guignon, B. Industrial Viability of the Hyperbaric Method to Store Perishable Foods at Room Temperature. J. Food Eng. 2017, 193, 76–85. [Google Scholar] [CrossRef]
- Segovia-Bravo, K.A.; Guignon, B.; Bermejo-Prada, A.; Sanz, P.D.; Otero, L. Hyperbaric Storage at Room Temperature for Food Preservation: A Study in Strawberry Juice. Innov. Food Sci. Emerg. Technol. 2012, 15, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.A.R.; Moreira, S.A.; Fidalgo, L.G.; Santos, M.D.; Queirós, R.P.; Delgadillo, I.; Saraiva, J.A. Food Preservation under Pressure (Hyperbaric Storage) as a Possible Improvement/Alternative to Refrigeration. Food Eng. Rev. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Gilbert, N. One-Third of Our Greenhouse Gas Emissions Come from Agriculture. Nature 2012, 1–4. [Google Scholar] [CrossRef]



| Enzyme(s) | Substrate(s) | Effect |
|---|---|---|
| Glycolytic enzymes | Glycogen | Lactic acid production resulting in pH drop |
| Nucleotide breakdown enzymes | ATP, ADP, AMP, IMP | Gradual production of Hx |
| Cathepsins | Proteins, peptides | Softening of tissue |
| Chymotrypsin, trypsin, carboxypeptidases | Proteins, peptides | Belly-bursting |
| Calpain | Myofibrillar proteins | Softening of tissue |
| Collagenases | Connective tissue | Softening and gaping of tissue |
| TMAO demethylase | TMAO | Formaldehyde production |
| Spoilage Bacteria | Spoilage Compound(s) Produced |
|---|---|
| Shewanella putrefaciens | TMA, H2S, CH3SH, (CH3)2S, Hx, and acids |
| Pseudomonas spp. | CH3SH, (CH3)2S, ketones, esters, aldehydes, NH3, and Hx |
| Photobacterium phosphoreum | TMA and Hx |
| Vibrionaceae | TMA and H2S |
| Enterobacteriaceae | TMA, H2S, ketones, esters, aldehydes, NH3, Hx, and acids |
| Lactic acid bacteria | H2S, ketones, esters, aldehydes, NH3, and acids |
| Yeast | Ketones, esters, aldehydes, NH3, and acids |
| Aerobic spoilers | NH3, acetic, butyric, and propionic acids |
| Anaerobic rods | Ketones, esters, aldehydes, and NH3 |
| Fish Species | Temperate Waters (Days) | Tropical Waters (Days) |
|---|---|---|
| Marine Species | 2–24 | 6–35 |
| Cod (Gadus morhua) | 9–15 | na |
| Hake (Merluccius merluccius) | 7–15 | na |
| Catfish | Na | 16–19 |
| Batfih (Ogcocephalus darwini) | Na | 21–24 |
| Halibut (Hippoglossus stenolepis) | 21–24 | na |
| Sardine (Sardina pilchardus) | 3–8 | 9–16 |
| Freshwater species | 9–17 | 6–40 |
| Catfish (Silurus glanis) | 12–13 | 15–27 |
| Trout (Oncorhynchus mykiss) | 9–11 | 16–24 |
| Perch (Perca spp.) | 8–17 | 13–32 |
| Tilapia (Oreochromis niloticus) | Na | 10–27 |
| Corvina (Argyrosomus regius) | Na | 30 |
| Species (Scientific Name) | Storage Conditions | Main Results | References |
|---|---|---|---|
| Atlantic Salmon (Salmo salar) | Superchilling: −1.0 °C up to 16 days Chilling: +4.0 °C up to 16 days Freezing: −40.0 °C up to 30 days | Superchilled salmon for nine days, without salting, presented the best results when compared with ice-chilled and frozen samples due to the reduction of biochemical quality degradation and a low/less degree of protein denaturation and structural damage. The highest production yield of salted salmon (in the weight of the salmon) was observed for the superchilling method at the ninth day of storage. | [45] |
| Superchilling: −1.4 °C and −3.6 °C up to 34 days Chilling: +4.0 °C up to 21 days Freezing: −40.0 °C up to 37 days Packaging: Vacuum | The storage time of vacuum-packed, superchilled salmon fillets can be doubled when compared to ice-chilled samples. The highest drip loss value and degree of protein and myosin denaturation for superchilled salmon stored at −1.4 °C. Better muscle hardness in superchilled samples stored at −3.6 °C and stable activity of cathepsins enzymes (B and B + L) in all salmon samples. | [46] | |
| Superchilling: −1.5 °C up to 28 days Chilling: +2.0 °C up to 21 days Packaging: MAP-CO2, N2 (CO2 compositions: 25%, 40%, 60%, 75%, and 90% with different gas-to-product ratios). | Extension of shelf life for superchilled product (using 90% CO2) salmon fillets, from 11 days to 22 days, compared to chilled/control samples. | [44] | |
| Superchilling: −2.0 °C air overwrap up to 24 days Chilling: +4.0 °C air overwrap up to 24 days Packaging: MAP—60% CO2, 40% N2 | Spoilage of MAP and air-stored salmon fillets after 10 days and seven days, respectively. Good microbial and sensorial quality during 24 days of storage for superchilled MAP salmon, compared to 21 days of superchilled salmon with air overwrap. | [47] | |
| Superchilling: −1.5 °C up to 28 days Packaging: Vacuum | The liquid loss value of the superchilled salmon fillets was significant after one day of storage and not significant between three and 14 days of storage, and after 21 days, this parameter decreased. The drip loss parameter of the superchilled salmon fillets remained without significant differences between one and 14 days of storage, but increased after 21 days. | [48] | |
| Cod (Gadus morhua) | Superchilling: −1.5 °C up to 15 days Chilling: +0.5 °C up to 15 days | The shelf-life of superchilled cod fillets increased by three days when compared to the chilling process, resulting in a total of 15 days of shelf-life. The shelf-life of cod samples stored at a chilled temperature (+0.5 °C) only increased from 12.5 to 14 days. | [49] |
| Superchilling: −1.0 °C up to 42 days Chilling: +4.0 °C up to 37 days Freezing: −21.0 °C for 36 days or −40.0 °C for 43 days Packaging: Vacuum | The superchilling technology combined with vacuum packaging prolonged the shelf-life of the cod fillets by several weeks when compared to the traditional chilling method. Drip loss and liquid loss parameters of superchilled cod fillets decreased and increased, respectively, compared to the chilled samples. | [38] | |
| Superchilling: −0.9 °C up to 21 days Chilling: +1.5 °C up to 21 days Packaging: MAP—50% CO2, 45% N2, 5% O2 | The shelf-life of fresh cod loins has been extended from nine days for ice-chilled storage to 16 or 17 days by superchilled storage. In addition, MAP combined with chilling and superchilling methods increased the shelf-life to 14 and 21 days, respectively. The superchilled MAP cod loins after seven days showed significant differences in muscle texture when compared to other storage methods due to the formation of ice crystals, when the storage temperature reached the freezing point of the fish. | [43] | |
| Superchilling: −2.0 °C or −3.6 °C up to four weeks Chilling: +0.0 °C up to four weeks Packaging: MAP—50% CO2, 45% N2, 5% O2 | The effect of brine (2.5 ± 1.0% NaCl) on cod loins was evaluated using the combined superchilling/MAP technology. The synergistic effect of superchilling/MAP extended the shelf-life of unbrined cod loins by 21 days (at −2.0 °C) instead of 14 to 15 days (at −2.0 °C) of the superchilled samples. The brined samples showed a shorter shelf-life compared to unbrined samples, especially in superchilling/MAP cod loins. | [50] | |
| Tilapia (Oreochromis niloticus) fillets | Superchilling: −1.0 °C Chilling: +1.0 °C Packaging: MAP—50% CO2, 50% N2 | The MAP tilapia fillets remained good for consumption at the microbiological level, even after 23 days of storage at both temperatures (−1.0 °C and +1.0 °C). However, some detrimental effects were observed in color, drip loss, and texture of samples. The best storage conditions, considering both sensorial evaluation and microbial counts, were 13–15 and 21 days for the chilled and superchilled tilapia fillets, respectively. | [51] |
| Type of Fish | Storage Time (Months) | ||
|---|---|---|---|
| −18 °C | −25 °C | −30 °C | |
| Fatty fish (sardines, salmon) | 4 | 8 | 12 |
| Lean fish (cod, haddock) | 8 | 18 | 24 |
| Flat fish (flounder, plaice) | 9 | 18 | 24 |
| Fish Product | Film/Coating Material | Active Compounds | Main Results | Reference |
|---|---|---|---|---|
| Beluga Sturegeon (Huso huso) fillet | Coating composed of 8.0% (w/v) whey protein concentrate, glycerol, and Tween 80 | Cinnamon essential oil (1.5% v/v) | Shelf-life extension by eight days (compared to uncoated control samples) by reducing lipid oxidation and microbial activity. | [70] |
| Bluefin tuna (Thunnus thynnus) slides | Film composed of 1.0% (w/v) fish myofibrillar protein, 25.0% (w/w) glycerol, and 2.0% (w/w) MTGase | Catechin-Kradon (Careya sphaerica Roxb.) extract (0.9% v/v) | Maintenance of sensorial qualities after eight days in comparison to the four days of the unwrapped control samples, due to the reduced microbial growth, lipid oxidation, and formation of metmyoglobin. | [71] |
| Freshwater rainbow trout (Oncorhynchus mykiss) fillets | Coating composed of 1.0% (w/w) carrageenan | Essential lemon oil (1.0% w/w) | The lemon essential oil incorporated in the carrageenan coating and applied to samples of rainbow trout fillets showed good antimicrobial activity and reduced lipid oxidation during the 15 days of storage at 4 °C. | [72] |
| Grass carp (Ctenopharyngodon idellus) fillets | Coating composed of 2.0% (w/v) chitosan | Glycerol monolaurate (0.1% and 0.3%) | After 20 days of storage at refrigerated temperature (4 °C), grass carp fillets maintained microbial safety, good quality, and sensorial properties. | [73] |
| Rainbow trout (Oncorhynchus mykiss) | Coating composed of 2.0% (w/v) chitosan, 1.0% acetic acid, 0.75% glycerol, and 0.2% Tween 80 | Cinnamon oil (1.5% v/v) | The coated rainbow trout during the refrigerated storage (4 °C) for 16 days maintained the overall quality and sensorial properties without significant microbial growth, compared to 12 days of shelf-life for the control samples. | [69] |
| Rainbow trout (Oncorhynchus mykiss) fillets | Film composed of 1.5% (w/w) quince seed mucilage (QSM), 35.0% (w/w) glycerol, and 0.1–0.2% (w/v) Tween 80 | Oregano or thyme essential oils (1.0, 1.5, or 2.0% v/v) | Shelf-life extension of rainbow trout fillets of up to 11 days compared to the control samples. | [74] |
| Rainbow trout (Oncorhynchus mykiss) slices | Film composed of 75.0% fish gelatin and 25.0% sodium alginate, glycerol, and Tween 80 | Oregano essential oil (OEO) (1.5% w/v) | The use of OEO gelatin-alginate film decreased microbial growth during the 15 days of the storage period compared to the control samples. | [75] |
| Red drum (Sciaenops ocellatus) fillets | Coating composed of 1.5% (w/v) chitosan, 25% glycerol, and 1.0% (v/v) acetic acid | Grape seed extract (0.2% w/v) or tea polyphenols (0.2% w/v) | Considering the results of microbiological, physicochemical (such as pH, TVB-N value, etc.), and sensorial analysis of red drum fillet samples immersed in the active coating, the shelf-life was extended by six to eight days in refrigerated storage (4 °C) when compared to the uncoated control samples. | [76] |
| Salmon slices * | Film composed of 8.0% (w/v) catfish-skin gelatin and chitosan, sorbitol, and glycerol | Clove essential oil (7.5% v/w) | Samples of salmon slices wrapped in edible films of gelatin-chitosan with clove essential oil showed an inhibitory action on spoilage and pathogenic microorganisms when stored at 2 °C, with a shelf-life of 11 days against nine days of the control samples. | [77] |
| Samples | Storage Conditions | Main Results | References |
|---|---|---|---|
| Cod fish fillets (Gadus morhua) | 22.8 MPa, at −3 °C for 36 days | An expert panel rated these samples with a similar or better quality than samples stored at −3 °C and at atmospheric pressure. | [86] |
| Dressed pollock (Pollachius pollachius) | 24.1 MPa, at 1 °C for 12 days | Microbial load with no changes during storage time. | [86] |
| Dressed cod fish (Gadus morhua) | 24.1 MPa, at 1 °C for 21 days | ||
| Carp * | 110 MPa/−8 °C and 170 MPa/−15 °C for 50 days | Enzymatic activity associated to nucleic acid degradation was slower than in samples stored at −8 °C and at atmospheric pressure. | [89] |
| Tilapia fillets (Oreochromis niloticus) | 100–200 MPa, at 25 °C for 12 h | Total plate counts remained stable at 100 MPa and showed a reduction of about two log units at 200 MPa. | [90] |
| Cape hake loins (Merluccius capensis) | 50 MPa, at 5 °C for seven days | Microbial counts and total volatile basic nitrogen content remained unaltered during storage. Drip losses, shear resistance, and whiteness increased, but, after cooking, these differences almost disappeared. | [91] |
| Atlantic salmon (Salmo salar) | 50–75 MPa, at 25–37 °C for 10 days | Initial microbial counts were reduced at 75 MPa, while no effect was observed at 50 MPa, and there was an inhibition at 60 MPa. No effect on color parameters. Increase of lipid oxidation state compared to refrigeration. | [92] |
| Atlantic mackerel (Scomber scombrus, L.) fillets | 50 MPa, at 5 °C for 12 days | Initial microbial counts were maintained or reduced. No significant lipid degradation was observed, and better fish quality indicators were observed (pH, TVB-N, drip loss, water holding capacity, and firmness after cooking) than under refrigeration. | [93] |
| Atlantic salmon (Salmo salar) | 40–60 MPa at 5–15 °C for 10 days | Microbial growth was slowed down with inactivation at 60 MPa. Low values of volatile base nitrogen at 60 MPa up to 15 days with stable trimethylamine-nitrogen. Increase of formaldehyde and dimethylamine-nitrogen content. | [94] |
| 50–75 MPa at 10–37 °C for 10 days | Initial activities of acid phosphatase, cathepsin B and D, and calpains decreased by increasing the storage temperature. A pronounced increase of myofibrillar fragmentation index at 75 MPa and 25 or 37 °C after 10 days. | [95] | |
| 60 MPa at 10 °C for 30 days | No variations in drip loss, water holding capacity, or myofibrillar fragmentation index. Low changes in muscle fibers, visible by scanning electron micrographs, and a decrease of the resilience property. No effect on fatty acids content, with a lower polyene index compared to refrigeration. Retention of fresh-like volatile compounds. | [96] | |
| 75 MPa at 25 °C for 30 days | Only resilience (textural property) was affected, decreasing after 30 days. Slower myofibrillar fragmentation index and no effect on fatty acids content, with a lower polyene index, compared to refrigeration. Retention of fresh-like volatile compounds. | [97] | |
| 60 MPa/10 °C and 75 MPa/25 °C for 30 days | Inhibition and inactivation the spoilage microorganisms. Reduction of surrogate pathogenic microorganism (Bacillus subtilis endospores, Escherichia coli, and Listeria innocua) counts. | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Silva, A.M.; Saraiva, J.A. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods 2021, 10, 780. https://doi.org/10.3390/foods10040780
Tavares J, Martins A, Fidalgo LG, Lima V, Amaral RA, Pinto CA, Silva AM, Saraiva JA. Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies. Foods. 2021; 10(4):780. https://doi.org/10.3390/foods10040780
Chicago/Turabian StyleTavares, Jéssica, Ana Martins, Liliana G. Fidalgo, Vasco Lima, Renata A. Amaral, Carlos A. Pinto, Ana M. Silva, and Jorge A. Saraiva. 2021. "Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies" Foods 10, no. 4: 780. https://doi.org/10.3390/foods10040780