Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer)
Abstract
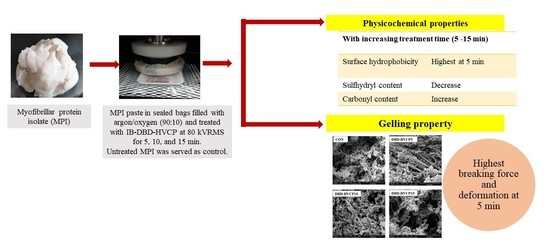
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Procurement and Preparation of Myofibrillar Protein Isolate (MPI)
2.3. The Impact of High Voltage Cold Plasma (HVCP) on Protein Oxidation (PT-OX) of MPI
2.4. The Impact of In-Bag Dielectric Barrier Discharge High Voltage Cold Plasma (IB-DBD-HVCP) on the Gelling Properties of MPI
2.4.1. Gel Preparation
2.4.2. Analyses
2.4.3. Microstructure
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of IB-DBD-HVCP on Asian Sea Bass (ASB) MPI
3.1.1. Total Carbonyl Content (TCC), Surface Hydrophobicity (SHP), and Total Sulfhydryl Group Content (TSHC)
3.1.2. Fourier Transform Infrared (FTIR) Spectra
3.2. Effect of IB-DBD-HVCP Treatment on the Gel Properties of MPI
3.2.1. Breaking Force (BF) and Deformation (DF)
3.2.2. Whiteness and Expressible Moisture Content (EMC)
3.2.3. Protein Patterns
3.2.4. Dynamic Rheological Properties
3.2.5. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulter, R.; Ledward, D.; Godber, S.; Hall, G.; Rowlands, B. Heat stability of fish muscle proteins. Int. J. Food Sci. Technol. 1985, 20, 203–217. [Google Scholar] [CrossRef]
- Chan, J.; Gill, T.; Paulson, A. The dynamics of thermal denaturation of fish myosins. Food Res. Int. 1992, 25, 117–123. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Nonthermal processes for shelf-life extension of seafoods: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.; Vicente, A. Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res. Int. 2010, 43, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge cold atmospheric plasma: Bacterial inactivation mechanism. J. Food Saf. 2019, 39, e12705. [Google Scholar] [CrossRef]
- Fukuda, S.; Kawasaki, Y.; Izawa, S. Ferrous chloride and ferrous sulfate improve the fungicidal efficacy of cold atmospheric argon plasma on melanized Aureobasidium pullulans. J. Biosci. Bioeng. 2019, 128, 28–32. [Google Scholar] [CrossRef]
- Hemmati, V.; Garavand, F.; Goudarzi, M.; Sarlak, Z.; Cacciotti, I.; Tiwari, B.K. Cold atmospheric-pressure plasma treatment of turmeric powder: Microbial load, essential oil profile, bioactivity and microstructure analyses. Int. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Shelf-life of refrigerated Asian sea bass slices treated with cold plasma as affected by gas composition in packaging. Int. J. Food Microbiol. 2020, 324, 108612. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Benjakul, S. Effect of high voltage cold atmospheric plasma processing on the quality and shelf-life of Pacific white shrimp treated with Chamuang leaf extract. Innov. Food Sci. Emerg. Technol. 2020, 64, 102435. [Google Scholar] [CrossRef]
- Liao, X.; Su, Y.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonca, A.; Keener, K. In-package decontamination of chicken breast using cold plasma technology: Microbial, quality and storage studies. Meat Sci. 2019, 159, 107942. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Olatunde, O.O.; Yesilsu, A.F. The combined effect of squid pen chitooligosaccharide and high voltage cold atmospheric plasma on the quality of Asian sea bass slices inoculated with Pseudomonas aeruginosa. Turk. J. Fish. Aquat. Sci. 2020, 21, 41–50. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Microbial diversity, shelf-life and sensory properties of Asian sea bass slices with combined treatment of liposomal encapsulated ethanolic coconut husk extract and high voltage cold plasma. LWT 2020, 134, 110232. [Google Scholar] [CrossRef]
- Garba, U.; Kaur, S. Protein isolates: Production, functional properties and application. Int. J. Curr. Res. Rev. 2014, 6, 35. [Google Scholar]
- Hamzeh, A.; Benjakul, S.; Senphan, T. Comparative study on antioxidant activity of hydrolysates from splendid squid (Loligo formosana) gelatin and protein isolate prepared using protease from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei). J. Food Sci. Technol. 2016, 53, 3615–3623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Essex, D.W.; Li, M.; Miller, A.; Feinman, R.D. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry 2001, 40, 6070–6075. [Google Scholar] [CrossRef] [PubMed]
- Soladoye, O.; Juárez, M.; Aalhus, J.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mayer, S.G.; Park, J.W. Gelation properties of tilapia fish protein isolate and surimi pre-and post-rigor: Rigor condition of tilapia FPI and surimi. Food Biosci. 2017, 17, 17–23. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Dielectric barrier discharge high voltage cold atmospheric plasma: An innovative nonthermal technology for extending the shelf-life of Asian sea bass slices. J. Food Sci. 2019, 84, 1871–1880. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Cold plasma combined with liposomal ethanolic coconut husk extract: A potential hurdle technology for shelf-life extension of Asian sea bass slices packaged under modified atmosphere. Innov. Food Sci. Emerg. Technol. 2020, 65, 102448. [Google Scholar] [CrossRef]
- Benjakul, S.; Seymour, T.A.; Morrissey, M.T.; An, H. Physicochemical changes in Pacific whiting muscle proteins during iced storage. J. Food Sci. 1997, 62, 729–733. [Google Scholar] [CrossRef]
- Zhou, A.; Benjakul, S.; Pan, K.; Gong, J.; Liu, X. Cryoprotective effects of trehalose and sodium lactate on tilapia (Sarotherodon nilotica) surimi during frozen storage. Food Chem. 2006, 96, 96–103. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; Xiong, Y.L. Physicochemical changes of myosin and gelling properties of washed tilapia mince as influenced by oxidative stress and microbial transglutaminase. J. Food Sci. Technol. 2015, 52, 3824–3836. [Google Scholar] [CrossRef] [Green Version]
- Raju, N.; Benjakul, S. Use of beta cyclodextrin to remove cholesterol and increase astaxanthin content in shrimp oil. Eur. J. Lipid Sci. Technol. 2020, 122, 1900242. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T. Effect of chitooligosaccharide from squid pen on gel properties of sardine surimi gel and its stability during refrigerated storage. Int. J. Food Sci. Technol. 2019, 54, 2831–2838. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Effect of serine protease inhibitor from squid ovary on gel properties of surimi from Indian mackerel. J. Texture Stud. 2017, 48, 541–549. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S. Comparative study on the effect of duck and hen egg albumens on proteolysis and gel property of sardine surimi. Int. J. Food Prop. 2017, 20, S2786–S2797. [Google Scholar] [CrossRef]
- Singh, A.; Prabowo, F.F.; Benjakul, S.; Pranoto, Y.; Chantakun, K. Combined effect of microbial transglutaminase and ethanolic coconut husk extract on the gel properties and in-vitro digestibility of spotted golden goatfish (Parupeneus heptacanthus) surimi gel. Food Hydrocoll. 2020, 109, 106107. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Combined effects of high voltage cold atmospheric plasma and antioxidants on the qualities and shelf-life of Asian sea bass slices. Innov. Food Sci. Emerg. Technol. 2019, 54, 113–122. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Effects of ozone-induced oxidation on the physicochemical properties of myofibrillar proteins recovered from bighead carp (Hypophthalmichthys nobilis). Food Bioprocess Technol. 2015, 8, 181–190. [Google Scholar] [CrossRef]
- Gonçalves, A.A. Ozone: An emerging technology for the seafood industry. Braz. Arch. Biol. Technol. 2009, 52, 1527–1539. [Google Scholar] [CrossRef]
- Refsgaard, H.H.; Tsai, L.; Stadtman, E.R. Modifications of proteins by polyunsaturated fatty acid peroxidation products. Proc. Natl. Acad. Sci. USA 2000, 97, 611–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delles, R.M.; Xiong, Y.L. The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Sci. 2014, 97, 181–188. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; de Alba, M.; Harrison, S.M.; Brunton, N.P.; Cullen, P.; Tiwari, B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT 2020, 118, 108697. [Google Scholar] [CrossRef]
- Benjakul, S.; Bauer, F. Physicochemical and enzymatic changes of cod muscle proteins subjected to different freeze–thaw cycles. J. Sci. Food Agric. 2000, 80, 1143–1150. [Google Scholar] [CrossRef]
- Attri, P.; Kumar, N.; Park, J.H.; Yadav, D.K.; Choi, S.; Uhm, H.S.; Kim, I.T.; Choi, E.H.; Lee, W. Influence of reactive species on the modification of biomolecules generated from the soft plasma. Sci. Rep. 2015, 5, 8221. [Google Scholar] [CrossRef] [Green Version]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- Liu, G.; Xiong, Y.L. Oxidatively induced chemical changes and interactions of mixed myosin, β-lactoglobulin and soy 7S globulin. J. Sci. Food Agric. 2000, 80, 1601–1607. [Google Scholar] [CrossRef]
- Nagy, P.; Winterbourn, C.C. Redox chemistry of biological thiols. In Advances in Molecular Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 183–222. [Google Scholar]
- Lanier, T.C.; Carvajal, P.; Yongsawatdigul, J. Surimi gelation chemistry. In Surimi and Surimi Seafood; Park, J.W., Ed.; Marcel Dekker: New York, NY, USA, 2000; Volume 2, pp. 237–265. [Google Scholar]
- Barth, A.; Zscherp, C. What vibrations tell about proteins. Q. Rev. Biophys. 2002, 35, 369. [Google Scholar] [CrossRef]
- Akyuz, S.; Akyuz, T.; Celik, O.; Atak, C. FTIR spectroscopy of protein isolates of salt-tolerant soybean mutants. J. Appl. Spectrosc. 2018, 84, 1019–1023. [Google Scholar] [CrossRef]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. Systematic FTIR spectroscopy study of the secondary structure changes in human serum albumin under various denaturation conditions. Biomolecules 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Lai, S.; Tong, H.; Lakey, P.S.; Shiraiwa, M.; Weller, M.G.; Pöschl, U.; Kampf, C.J. Release of free amino acids upon oxidation of peptides and proteins by hydroxyl radicals. Anal. Bioanal. Chem. 2017, 409, 2411–2420. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.T.; Ho, M.L.; Jiang, S.H.; Lo, L.; Chen, H.C. Color and quality of mackerel surimi as affected by alkaline washing and ozonation. J. Food Sci. 1998, 63, 652–655. [Google Scholar] [CrossRef]
- Balange, A.; Benjakul, S. Enhancement of gel strength of bigeye snapper (Priacanthus tayenus) surimi using oxidised phenolic compounds. Food Chem. 2009, 113, 61–70. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. High voltage cold atmospheric plasma: Antibacterial properties and its effect on quality of Asian sea bass slices. Innov. Food Sci. Emerg. Technol. 2019, 52, 305–312. [Google Scholar] [CrossRef]
- Kjærsgård, I.V.; Nørrelykke, M.R.; Baron, C.P.; Jessen, F. Identification of carbonylated protein in frozen rainbow trout (Oncorhynchus mykiss) fillets and development of protein oxidation during frozen storage. J. Agric. Food Chem. 2006, 54, 9437–9446. [Google Scholar]
- Park, D.; Xiong, Y.L.; Alderton, A.L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chem. 2007, 101, 1239–1246. [Google Scholar] [CrossRef]
- Stagsted, J.; Bendixen, E.; Andersen, H.J. Identification of specific oxidatively modified proteins in chicken muscles using a combined immunologic and proteomic approach. J. Agric. Food Chem. 2004, 52, 3967–3974. [Google Scholar] [CrossRef]
- Baron, C.P.; KjÆrsgård, I.V.; Jessen, F.; Jacobsen, C. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J. Agric. Food Chem. 2007, 55, 8118–8125. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Improvement of gel properties of sardine (Sardinella albella) surimi using coconut husk extracts. Food Hydrocoll. 2015, 51, 146–155. [Google Scholar] [CrossRef]
- Ross, A.C.; Bell, G.; Halling, P.J. Effect of pH on rate of interfacial inactivation of serine proteases in aqueous–organic systems. Biotechnol. Bioeng. 2000, 67, 498–503. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of deacetylation of konjac glucomannan on Alaska Pollock surimi gels subjected to high-temperature (120 °C) treatment. Food Hydrocoll. 2015, 43, 125–131. [Google Scholar] [CrossRef]
- Permentier, H.P.; Bruins, A.P. Electrochemical oxidation and cleavage of proteins with on-line mass spectrometric detection: Development of an instrumental alternative to enzymatic protein digestion. J. Am. Soc. Mass Spectrom. 2004, 15, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef]
- Shitole, S.S.; Balange, A.K.; Gangan, S.S. Use of seaweed (Sargassum tenerrimum) extract as gel enhancer for lesser sardine (Sardinella brachiosoma) surimi. Int. Aquat. Res. 2014, 6, 55. [Google Scholar] [CrossRef]
- Ngo, V.P.; Morioka, K.; Itoh, Y. Microstructure of white croaker surimi protein gels set at low temperature under the inhibition of the polymerization and degradation of protein. J. Biol. Sci. 2010, 10, 499–506. [Google Scholar]






| Samples | Total Carbonyl Content (nmol/g Protein) | Surface Hydrophobicity | Total Sulfhydryl Content (µmol/g Protein) |
|---|---|---|---|
| CON | 1.16 ± 0.01 d | 1165.4 ± 2.21 c | 7.94 ± 0.20 a |
| DBD-HVCP5 | 2.39 ± 0.07 c | 1484.6 ± 0.97 a | 6.53 ± 0.10 b |
| DBD-HVCP10 | 4.76 ± 0.02 b | 1380 ± 1.04 b | 5.28 ± 0.02 c |
| DBD-HVCP15 | 7.93 ± 0.01 a | 947.84 ± 1.41 d | 2.94 ± 0.05 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunde, O.O.; Singh, A.; Shiekh, K.A.; Nuthong, P.; Benjakul, S. Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer). Foods 2021, 10, 326. https://doi.org/10.3390/foods10020326
Olatunde OO, Singh A, Shiekh KA, Nuthong P, Benjakul S. Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer). Foods. 2021; 10(2):326. https://doi.org/10.3390/foods10020326
Chicago/Turabian StyleOlatunde, Oladipupo Odunayo, Avtar Singh, Khursheed Ahmad Shiekh, Pornpot Nuthong, and Soottawat Benjakul. 2021. "Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer)" Foods 10, no. 2: 326. https://doi.org/10.3390/foods10020326