Stability of Bioactive Compounds in Olive-Pomace Oil at Frying Temperature and Incorporation into Fried Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Assays
2.3.1. Thermoxidation
2.3.2. Discontinuous Frying
2.4. Analytical Procedures
2.4.1. Oil Analyses
2.4.2. Food Analyses
- (a)
- Moisture contentMoisture content of initial and fried foods was determined gravimetrically by drying in an oven according to Official AOAC method 7.003 [28].
- (b)
- Lipid contentInitial and fried foods were frozen, freeze-dried, and ground. The total lipids were obtained by Söxhlet extraction with hexane for 6 hours, according to method UNE 55-062-80 [29].
- (c)
- Food lipid analysesThe lipids extracted from the initial and fried foods were analyzed following the same methods described in 2.4.1.
2.5. Statistical Analyses
3. Results and Discussion
3.1. Characterization and Quality of Unused Oils
3.2. Thermoxidation Assay
3.3. Discontinuous Frying
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Márquez-Ruiz, G.; Holgado, F. Frying performance of olive-extracted oils. Grasas Aceites 2018, 69, e264. [Google Scholar] [CrossRef]
- Boskou, D. Olive fruit, table olives, and olive oil bioactive constituents. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; Academic Press and AOCS Press: Urnana, IL, USA, 2015; pp. 1–30. ISBN 9781630670412. [Google Scholar]
- Chiou, A.; Kalogeropoulos, N. Virgin olive oil as frying oil. Comp. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrikopoulos, N.K.; Dedoussis, G.V.Z.; Falirea, A.; Kalogeropoulos, N.; Hatzinikola, H.S. Deterioration of natural antioxidant species of vegetable edible oils during the domestic deep-frying and pan-frying of potatoes. Int. J. Food Sci. Nutr. 2002, 53, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; Dobarganes, M.C.; Velasco, J.; Romero, C. Influence of thermal treatments simulating cooking processes on the polyphenol content of virgin olive oil. J. Agric. Food Chem. 2002, 50, 5962–5967. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How heating affects extra virgin olive oil quality indexes and chemical composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, I.; Triki, M.; Matthäus, B.; Bouaziz, M. A comparative study on formation of polar components, fatty acids and sterols during frying of refined olive pomace oil pure and its blend coconut oil. J. Agric. Food Chem. 2018, 66, 3514–3523. [Google Scholar] [CrossRef]
- Hammouda, I.B.; Márquez-Ruiz, G.; Holgado, F.; Freitas, F.; Da Silva, M.G.; Bouaziz, M. Comparative study of polymers and total polar compounds as indicators of refined oil degradation during frying. Eur. Food Res. Technol. 2019, 245, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.M.; Capocasale, M.; Macrì, R.; Caracciolo, M.; Zappia, C.; Poiana, M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. LWT 2020, 117, 108631. [Google Scholar] [CrossRef]
- Regulation, H. Commission Regulation (EEC) No. 2568/91 of 11 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis Official Journal L 248, 5 September 1991. Offic. JL 1991, 248, 1–83. [Google Scholar]
- Sánchez-Gutiérrez, C.A.; Ruiz-Méndez, M.V.; Jiménez-Castellano, M.R.; Lucero, M.J. Influence of refining processes on content of bioactive compounds, rheology and texture of olive pomace oil for use in topical formulations. Eur. J. Lipid Sci. Technol. 2017, 119, 1600408. [Google Scholar] [CrossRef] [Green Version]
- Nergiz, C.; Çelikkale, D. The effect of consecutive steps of refining on squalene content of vegetable oils. J. Food Sci. Technol. 2011, 48, 382–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateos, R.; Sarriá, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. 2020, 60, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miana, M.; Jurado-Lopez, R.; Martínez-Martínez, E.; Gómez-Hurtado, N.; Delgado, C.; Bartolomé, M.V.; San Román, J.A.; Cordova, C.; Lahera, V.; et al. DIOL triterpenes block profibrotic effects of angiotensin II and protect from cardiac hypertrophy. PLoS ONE 2012, 7, e41545. [Google Scholar] [CrossRef] [Green Version]
- Allouche, Y.; Beltrán, G.; Gaforio, J.J.; Uceda, M.; Mesa, M.D. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol. 2010, 48, 2885–2890. [Google Scholar] [CrossRef]
- Agra, L.C.; Lins, M.P.; da Silva Marques, P.; Smaniotto, S.; Bandeira de Melo, C.; Lagente, V.; Barreto, E. Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice. Eur. J. Pharmacol. 2016, 780, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.H.; Solís-Gutiérrez, M.; Rojas-Molina, J.I.; Rivero-Cruz, F. Role of nitric oxide and hydrogen sulfide in the vasodilator effect of ursolic acid and uvaol from black cherry Prunus serotina fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef] [Green Version]
- Suárez Montenegro, Z.J.; Álvarez-Rivera, G.; Sánchez-Martínez, J.D.; Gallego, R.; Valdés, A.; Bueno, M.; Cifuentes, A.; Ibáñez, E. Neuroprotective Effect of Terpenoids Recovered from Olive Oil By-Products. Foods 2021, 10, 1507. [Google Scholar] [CrossRef]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. 2004, 229, 215–226. [Google Scholar] [CrossRef]
- Fernández-Arche, A.; Marquez-Martín, A.; de la Puerta Vazquez, R.; Perona, J.S.; Terencio, C.; Pérez-Camino, C.; Ruiz-Gutierrez, V. Long-chain fatty alcohols from pomace olive oil modulate the release of proinflammatory mediators. J. Nutr. Biochem. 2009, 20, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Chiou, A.; Kalogeropoulos, N.; Boskou, G.; Salta, F.N. Migration of health promoting microconstituents from frying vegetable oils to French fries. Food Chem. 2012, 133, 1255–1263. [Google Scholar] [CrossRef]
- de Alzaa, F.; Guillaume, C.; Ravetti, L. Evaluation of Chemical and Nutritional Changes in Chips, Chicken Nuggets, and Broccoli after Deep-Frying with Extra Virgin Olive Oil, Canola, and Grapeseed Oils. J. Food Quality 2021, 2021, 7319013. [Google Scholar] [CrossRef]
- Barrera-Arellano, D.; Márquez-Ruiz, G.; Dobarganes, M.C. A simple procedure to evaluate the performance of fats and oils at frying temperatures. Grasas Aceites 1997, 48, 231–235. [Google Scholar] [CrossRef] [Green Version]
- IOC (International Olive Council): Madrid, Spain. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/Method-COI-T.20-Doc.-No-31-2012.pdf (accessed on 23 November 2021).
- Pérez-Camino, M.C.; Cert, A. Quantitative determination of hydroxyl pentacyclic triterpene acids in vegetable oils. J. Agric. Food Chem. 1999, 47, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- IUPAC International Union of Pure and Applied Chemistry. Standard Methods for the Analysis of Oil and Derivatives, 7th ed.; Dieffenbacher, A., Pocklington, W.D., Eds.; Blackwell Scientific Publications: Oxford, UK, 1992; Volume (Suppl. S1), ISBN 0-632-03337-. [Google Scholar]
- Dobarganes, M.C.; Pérez-Camino, M.C.; Márquez-Ruiz, G. High performance size exclusion chromatography of polar compounds in heated and non-heated fats. Fat Sci. Technol. 1998, 90, 308–311. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC INTERNATIONAL; AOAC International: Rockville, MD, USA, 1995; ISBN 13978-0935584899. [Google Scholar]
- AENOR Asociación Española de Normalización (Spanish Association for Standardization). Catálogo de Normas UNE; Asociación Española de Normalización: Madrid, Spain, 1991; ISBN 8486688469. [Google Scholar]
- Ramos-Hinojosa, A.E.; Ruiz-Méndez, M.V. Two phases olive pomace storaged in ponds. Grasas Aceites 2004, 55, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Méndez, M.V.; Dobarganes, M.C. Combination of chromatographic techniques for the analysis of complex deodoriser distillates from an edible oil refining process. Food Chem. 2007, 103, 1502–1507. [Google Scholar] [CrossRef]
- Rodrigues Machado, E.; Marmesat, S.; Abrantes, S.; Dobarganes, M.C. Uncontrolled variables in frying studies: Differences in repeatability between thermoxidation and frying experiments. Grasas Aceites 2007, 58, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Firestone, D. Regulation of frying fats and oils. In Deep Frying: Chemistry, Nutrition and Practical Applications; Erickson, M.D., Ed.; Academic Press and AOCS Press: Champaign, IL, USA, 2007; pp. 373–385. ISBN 9781893997929. [Google Scholar]
- Berdeaux, O.; Marmesat, S.; Velasco, J.; Dobarganes, M.C. Apparent and quantitative loss of fatty acids and triacylglycerols at frying temperatures. Grasas Aceites 2012, 63, 284–289. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Valk, I.; van Andel, V.; Friedrichs, S.; Lütjohann, D.; Hrncirik, K.; Trautwein, E.A. Thermal stability of plant sterols and formation of their oxidation products in vegetable oils and margarines upon controlled heating. Chem. Phys. Lipids 2017, 207, 99–107. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Rudzińska, M.; Siger, A.; Gramza-Michałowska, A.; Kobus-Cisowska, J. Thermo-Oxidation of Phytosterol Molecules in Rapeseed Oil during Heating: The Impact of Unsaturation Level of the Oil. Foods 2021, 10, 50. [Google Scholar] [CrossRef]
- Blekas, G.; Boskou, D. Phytosterols and frying oils. In Frying of Food: Oxidation, Nutrient and Non-Nutrient Antioxidants, Biologically Active Compounds and High Temperatures, 2nd ed.; Boskou, D., Elmadfa, I., Eds.; CRC Press: Boca Ratón, FL, USA, 2016; pp. 225–249. ISBN 9780367383176. [Google Scholar]
- Barrera-Arellano, D.; Ruiz-Méndez, M.V.; Márquez-Ruiz, G.; Dobarganes, C. Loss of tocopherols and formation of degradation compounds in triacylglycerol model systems heated at high temperature. J. Sci. Food Agric. 1999, 79, 1923–1928. [Google Scholar] [CrossRef]
- Barrera-Arellano, D.; Ruiz-Méndez, V.; Velasco, J.; Márquez-Ruiz, G.; Dobarganes, C. Loss of tocopherols and formation of degradation compounds at frying temperatures in oils differing in degree of unsaturation and natural antioxidant content. J. Sci. Food Agric. 2002, 82, 1696–1702. [Google Scholar] [CrossRef]
- Marmesat, S.; Morales, A.; Velasco, J.; Dobarganes, M.C. Action and fate of natural and synthetic antioxidants during frying. Grasas Aceites 2010, 61, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Ruiz, G.; Ruiz-Méndez, M.V.; Velasco, J. Antioxidants in frying: Analysis and evaluation of efficacy. Eur. J. Lipid Sci. Technol. 2014, 116, 1441–1450. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, N.; Ito, J.; Kato, S.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Significance of squalene in rice bran oil and perspectives on squalene oxidation. J. Nutr. Sci. Vitaminol. 2019, 65 (Suppl. 62), 62–66. [Google Scholar] [CrossRef] [Green Version]
- Manzi, P.; Panüli, G.; Esti, M.; Pizzoferrato, L. Natural Antioxidants in the Unsaponifiable Fraction of Virgin Olive Oils from Different Cultivars. J. Sci. Food Agric. 1998, 77, 115–120. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Márquez-Ruiz, G.; Velasco, J. Interactions between fat and food during deep-frying. Eur. J. Lipid Sci. Technol. 2000, 102, 521–528. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Márquez-Ruiz, G.; Ruiz-Méndez, M.V.; Dobarganes, M.C. Lipid changes during frying of frozen prefried foods. J. Food Sci. 1991, 56, 1644–1650. [Google Scholar] [CrossRef]
- Romero, A.; Bastida, S.; Sánchez-Muniz, F.J. Cyclic fatty acid monomer formation in domestic frying of frozen foods in sunflower oil and high oleic acid sunflower oil without oil replenishment. Food Chem. Toxicol. 2006, 44, 1674–1681. [Google Scholar] [CrossRef]
- Enríquez-Fernández, B.E.; Álvarez de la Cadena y Yañez, L.; Sosa-Morales, M.E. Comparison of the stability of palm olein and a palm olein/canola oil blend during deep-fat frying of chicken nuggets and French fries. Int. J. Food Sci. Technol. 2011, 46, 1231–1237. [Google Scholar] [CrossRef]
- Soto-Jover, S.; Boluda-Aguilar, M.; Eznoz-Nicuesa, A.; Iguaz-Gainza, A.; López-Gómez, A. Texture, oil adsorption and safety of the European style croquettes manufactured at industrial scale. Food Eng. Rev. 2016, 8, 181–200. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N.; Salta, F.N.; Efstathiou, P.; Andrikopoulos, N.K. Pan-frying of French fries in three different edible oils enriched with olive leaf extract: Oxidative stability and fate of microconstituents. LWT 2009, 42, 1090–1097. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Grigorakis, D.; Mylona, A.; Falirea, A.; Andrikopoulos, N.K. Dietary evaluation of vegetables pan-fried in virgin olive oil following the Greek traditional culinary practice. Ecol. Food Nutr. 2006, 45, 105–123. [Google Scholar] [CrossRef]
- Salta, F.N.; Kalogeropoulos, N.; Karavanou, N.; Andrikopoulos, N.K. Distribution and retention of phytosterols in frying oils and fried potatoes during repeated deep and pan frying. Eur. Food Res. Technol. 2008, 227, 391–400. [Google Scholar] [CrossRef]
- Tabee, E.; Azadmard-Damirchi, S.; Jägerstad, M.; Dutta, P.C. Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. J. Food Compos. Anal. 2008, 21, 169–177. [Google Scholar] [CrossRef]
- Sébédio, J.L.; Dobarganes, M.C.; Márquez-Ruiz, G.; Wester, I.; Christie, W.W.; Dobson, G.; Zwobada, F.; Chardigny, J.M.; Mairot, T.; Lahtinen, R. Industrial production of crisps and prefried French fries using sunflower oil. Grasas Aceites 1996, 47, 5–13. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Mylona, A.; Chiou, A.; Ioannou, M.S.; Andrikopoulos, N.K. Retention and distribution of natural antioxidants (α-tocopherol, polyphenols and terpenic acids) after shallow frying of vegetables in virgin olive oil. LWT 2007, 40, 1008–1017. [Google Scholar] [CrossRef]
- .Spanova, M.; Daum, G. Squalene—biochemistry, molecular biology, process biotechnology, and applications. Eur. J. Lipid Sci. Tech. 2011, 113, 1299–1320. [Google Scholar] [CrossRef]
- Ramírez-Torres, A.; Gabas, C.; Barranquero, C. Squalene: Current Knowledge and Potential Therapeutical Uses; Osada, J., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2010; ISBN 978-1-61728-974-3. [Google Scholar]
- Kalogeropoulos, N.; Andrikopoulos, N.K. Squalene in fats and oils used for the domestic and commercial frying of potatoes. Int. J. Food Sci. Nutr. 2004, 55, 125–129. [Google Scholar] [CrossRef]


| OPO1 | OPO2 | OPO3 | |
|---|---|---|---|
| Fatty Acids (wt%) C16:0 | 12.02 ± 0.01c | 11.10 ± 0.00a | 11.42 ± 0.01b |
| C16:1 | 0.87 ± 0.00c | 0.72 ± 0.00a | 0.83 ± 0.01b |
| C18:0 | 2.94 ± 0.01b | 2.75 ± 0.01a | 2.84 ± 0.01b |
| C18:1 | 70.36 ± 0.01a | 73.29 ± 0.04c | 72.69 ± 0.01b |
| C18:2 | 11.46 ± 0.01c | 9.19 ± 0.01a | 9.55 ± 0.01b |
| C18:3 | 0.72 ± 0.02c | 0.65 ± 0.00a | 0.69 ± 0.01b |
| C20:0 | 0.46 ± 0.01b | 0.40 ± 0.00a | 0.44 ± 0.02b |
| C20:1 | 0.31 ± 0.01a | 0.31 ± 0.00a | 0.35 ± 0.00b |
| C22:0 | 0.12 ± 0.10a | 0.18 ± 0.01a | 0.19 ± 0.00b |
| C24:0 | nd | 0.07 ± 0.06a | 0.07 ± 0.00a |
| Others | 0.24 ± 0.01a | 1.07 ± 0.00c | 0.60 ± 0.00b |
| Trans C18:1 | 0.21 ± 0.00a | 0.23 ± 0.00b | 0.27 ± 0.00c |
| Trans C18:2 | nd | 0.06 ± 0.00a | 0.07 ± 0.00a |
| Σ Trans | 0.21 ± 0.00a | 0.29 ± 0.00b | 0.34 ± 0.00c |
| Polar Compounds (wt%) Total | 7.6 ± 0.2a | 10.6 ± 2.1b | 10.1 ± 0.4b |
| Oligomers | nd | nd | nd |
| Dimers | 0.6 ± 0.1a | 0.8 ± 0.0a | 0.7 ± 0.1a |
| Oxidized Triacylglycerols | 0.9 ± 0.1a | 1.3 ± 0.3a | 1.3 ± 0.2a |
| Diacylglycerols | 5.4 ± 0.1a | 7.5 ± 1.6a | 7.3 ± 0.3a |
| Monoacylglycerols | 0.2 ± 0.0a | 0.2 ± 0.0a | 0.2 ± 0.0a |
| Free Fatty Acids * | 0.5 ± 0.0a | 0.8 ± 0.1b | 0.6 ± 0.1a |
| Bioactive compounds (mg/kg) | |||
| Tocopherols | 496 ± 13c | 344 ± 6b | 310 ± 4a |
| Squalene | 1062 ± 32b | 3699 ± 58c | 387 ± 13a |
| Sterols | 2515 ± 26a | 3021 ± 23b | 3438 ± 17c |
| Aliphatic alcohols | 1875 ± 56a | 1815 ± 5a | 2136 ± 33b |
| Erythrodiol | 616 ± 13ab | 585 ± 39a | 660 ± 18b |
| Oleanolic Acid | 201 ± 10a | 425 ± 45b | 358 ± 52b |
| Polar Compounds (wt%) | OPO1 | OPO2 | OPO3 |
|---|---|---|---|
| Total | 33.1 ± 3.5a | 34.8 ± 1.7a | 38.0 ± 2.3a |
| Oligomers | 7.4 ± 1.1a | 8.3 ± 0.3a | 8.8 ± 0.5a |
| Dimers | 10.3 ± 0.4a | 9.0 ± 0.7a | 10.2 ± 0.7a |
| Oxidized Triacylglycerols | 9.5 ± 2.3a | 11.6 ± 0.4a | 11.2 ± 0.4a |
| Diacylglycerols | 5.2 ± 0.2a | 5.3 ± 0.3a | 7.0 ± 0.5b |
| Monoacylglycerols | 0.1 ± 0.1a | 0.2 ± 0.0a | 0.2 ± 0.0a |
| Free Fatty Acids * | 0.4 ± 0.0a | 0.5 ± 0.0a | 0.5 ± 0.1a |
| Oil | 0 h | 2 h | 8 h | 15 h | 20 h |
|---|---|---|---|---|---|
| OPO1 | |||||
| C16:0 | 12.02 ± 0.01a | 12.84 ± 0.14b | 13.06 ± 0.02b | 13.37 ± 0.08c | 13.64 ± 0.14d |
| C16:1 | 0.87 ± 0.00a | 0.93 ± 0.02b | 0.97 ± 0.00c | 0.97 ± 0.00c | 0.97 ± 0.01c |
| C18:0 | 2.94 ± 0.01bc | 2.83 ± 0.02a | 2.89 ± 0.02b | 2.96 ± 0.03cd | 3.01 ± 0.04d |
| C18:1 | 70.36 ± 0.01d | 69.76 ± 0.27c | 69.53 ± 0.09bc | 69.35 ± 0.04ab | 69.15 ± 0.11a |
| C18:2 | 11.46 ± 0.01d | 10.93 ± 0.37d | 10.01 ± 0.06c | 9.09 ± 0.25b | 8.34 ± 0.41a |
| C18:3 | 0.72 ± 0.01d | 0.59 ± 0.04c | 0.49 ± 0.01b | 0.40 ± 0.02a | 0.34 ± 0.03a |
| C20:0 | nd | 0.45 ± 0.01a | 0.51 ± 0.00b | 0.51 ± 0.00b | 0.53 ± 0.00c |
| C20:1 | 0.31 ± 0.00a | 0.31 ± 0.01a | 0.36 ± 0.01b | 0.37 ± 0.02bc | 0.38 ± 0.01c |
| C22:0 | 0.12 ± 0.10a | 0.18 ± 0.01a | 0.21 ± 0.01a | 0.21 ± 0.01a | 0.23 ± 0.01a |
| C24:0 | nd | nd | 0.10 ± 0.02b | 0.09 ± 0.00b | 0.09 ± 0.00b |
| Others | 0.24 ± 0.01a | 1.10 ± 0.52b | 1.40 ± 0.10bc | 2.10 ± 0.17cd | 2.67 ± 0.32d |
| tC18:1 | 0.21 ± 0.00a | 0.37 ± 0.02b | 0.40 ± 0.01b | 0.50 ± 0.02c | 0.57 ± 0.04d |
| tC18:2 | nd | nd | 0.07 ± 0.00b | 0.08 ± 0.01bc | 0.08 ± 0.01c |
| ∑trans | 0.21 ± 0.00a | 0.37 ± 0.02b | 0.47 ± 0.01c | 0.58 ± 0.03d | 0.66 ± 0.03e |
| OPO2 | |||||
| C16:0 | 11.10 ± 0.00a | 11.26 ± 0.03b | 11.68 ± 0.05c | 12.17 ± 0.05d | 12.27 ± 0.06d |
| C16:1 | 0.72 ± 0.00a | 0.73 ± 0.00b | 0.74 ± 0.01c | 0.75 ± 0.00c | 0.73 ± 0.00b |
| C18:0 | 2.75 ± 0.01a | 2.78 ± 0.02a | 2.88 ± 0.02b | 3.00 ± 0.02c | 3.02 ± 0.03c |
| C18:1 | 73.29 ± 0.04b | 73.74 ± 0.04c | 74.15 ± 0.03d | 74.47 ± 0.01e | 73.02 ± 0.10a |
| C18:2 | 9.19 ± 0.01e | 8.97 ± 0.09d | 7.96 ± 0.10c | 6.91 ± 0.09b | 6.07 ± 0.09a |
| C18:3 | 0.65 ± 0.00e | 0.61 ± 0.02d | 0.48 ± 0.01c | 0.36 ± 0.01b | 0.28 ± 0.01a |
| C20:0 | 0.40 ± 0.00a | 0.40 ± 0.00a | 0.41 ± 0.01a | 0.44 ± 0.00b | 0.46 ± 0.01b |
| C20:1 | 0.31 ± 0.00a | 0.32 ± 0.01a | 0.37 ± 0.00b | 0.38 ± 0.00c | 0.37 ± 0.00b |
| C22:0 | 0.18 ± 0.01ab | 0.17 ± 0.00a | 0.19 ± 0.01abc | 0.21 ± 0.01c | 0.20 ± 0.02bc |
| C24:0 | 0.07 ± 0.00a | 0.06 ± 0.00a | 0.07 ± 0.00a | 0.08 ± 0.00b | 0.08 ± 0.00b |
| Others | 1.07 ± 0.06b | 0.63 ± 0.06a | 0.67 ± 0.06a | 0.70 ± 0.00a | 2.83 ± 0.06c |
| tC18:1 | 0.23 ± 0.00a | 0.25 ± 0.01a | 0.35 ± 0.02b | 0.48 ± 0.01c | 0.56 ± 0.03d |
| tC18:2 | 0.06 ± 0.00a | 0.06 ± 0.00a | 0.06 ± 0.01ab | 0.08 ± 0.01c | 0.07 ± 0.00bc |
| ∑trans | 0.29 ± 0.00a | 0.31 ± 0.01a | 0.41 ± 0.03b | 0.55 ± 0.01c | 0.63 ± 0.02d |
| OPO3 | |||||
| C16:0 | 11.42 ± 0.01a | 11.52 ± 0.02a | 11.81 ± 0.13ab | 12.09 ± 0.36bc | 12.52 ± 0.08c |
| C16:1 | 0.83 ± 0.01a | 0.85 ± 0.01ab | 0.85 ± 0.00ab | 0.85 ± 0.01b | 0.84 ± 0.01ab |
| C18:0 | 2.84 ± 0.01a | 2.84 ± 0.01a | 2.92 ± 0.04ab | 2.99 ± 0.09bc | 3.09 ± 0.02c |
| C18:1 | 72.69 ± 0.01b | 72.81 ± 0.01b | 73.13 ± 0.10c | 73.30 ± 0.17c | 72.30 ± 0.17a |
| C18:2 | 9.55 ± 0.01c | 9.43 ± 0.05c | 8.68 ± 0.27bc | 8.10 ± 0.71b | 6.59 ± 0.22a |
| C18:3 | 0.69 ± 0.01d | 0.67 ± 0.01cd | 0.56 ± 0.04bc | 0.49 ± 0.08b | 0.33 ± 0.02a |
| C20:0 | 0.44 ± 0.02a | 0.47 ± 0.00ab | 0.48 ± 0.00b | 0.49 ± 0.02b | 0.50 ± 0.00b |
| C20:1 | 0.35 ± 0.00a | 0.35 ± 0.01a | 0.38 ± 0.01b | 0.39 ± 0.01b | 0.38 ± 0.01b |
| C22:0 | 0.19 ± 0.00a | 0.19 ± 0.01a | 0.19 ± 0.00a | 0.20 ± 0.01a | 0.23 ± 0.01b |
| C24:0 | 0.07 ± 0.00a | 0.07 ± 0.00a | 0.08 ± 0.00b | 0.08 ± 0.01b | 0.09 ± 0.01b |
| Others | 0.60 ± 0.00a | 0.47 ± 0.06a | 0.50 ± 0.00a | 0.53 ± 0.06a | 2.53 ± 0.32b |
| tC18:1 | 0.27 ± 0.00a | 0.28 ± 0.01a | 0.35 ± 0.03ab | 0.41 ± 0.08b | 0.55 ± 0.02c |
| tC18:2 | 0.07 ± 0.00a | 0.07 ± 0.00a | 0.07 ± 0.00a | 0.07 ± 0.01a | 0.07 ± 0.00a |
| ∑trans | 0.34 ± 0.00a | 0.35 ± 0.01a | 0.42 ± 0.03ab | 0.49 ± 0.09b | 0.62 ± 0.02c |
| Sterol (% on Total) | 0 h | 2 h | 8 h | 15 h | 20 h |
|---|---|---|---|---|---|
| OPO1 | |||||
| Chol | 0.34 ± 0.03a | 0.63 ± 0.19a | 1.41 ±1.06a | 2.18 ± 2.04a | 1.86 ± 2.80a |
| Camp | 3.30 ± 0.04a | 3.76 ± 0.14a | 3.26 ±0.17a | 3.20 ± 0.05a | 3.83 ± 0.85a |
| Stigm | 1.55 ± 0.03a | 2.88 ± 0.79b | 1.15 ±0.06a | 1.14 ± 0.02a | 1.32 ± 0.25a |
| Δ7-Camp | 1.20 ± 0.00a | 1.33 ± 0.00b | 1.30 ±0.03b | 1.32 ± 0.02b | 1.50 ± 0.00c |
| Cler | 0.00 ± 0.07a | 0.08 ±0.07a | 0.11 ±0.03a | 0.00 ± 0.03a | 0.05 ± 0.29a |
| β-Sito | 85.46 ± 0.06a | 85.09 ± 0.20a | 86.14 ± 0.10a | 85.86 ± 0.00a | 85.85 ± 0.09a |
| Sitos | 2.11 ± 0.04a | 3.20 ± 1.26ab | 2.83 ± 1.03ab | 2.96 ± 1.97ab | 3.44 ± 2.32b |
| Δ5-Aven | 1.66 ± 0.07b | 1.01 ± 0.03ab | 1.43 ± 0.22ab | 1.19 ± 0.36a | 1.44 ± 0.58ab |
| Δ5,24-Sti | 2.07 ± 0.02a | 1.12 ± 0.08a | 1.71 ± 0.07a | 1.72 ± 0.04a | 1.94 ± 0.28a |
| Δ7-Stigmn | 0.62 ± 0.02a | 0.64 ± 0.85b | 0.45 ± 0.15a | 0.36 ± 0.01a | 0.48 ± 0.30a |
| Δ7-Aven | 0.30 ± 0.03a | 0.00 ± 0.87a | 0.00 ± 0.04a | 0.10 ± 0.03a | 0.00 ± 0.16a |
| Total (mg/kg) | 2515 ± 26a | 2387 ± 34b | 2155 ± 34c | 2045 ± 56 d | 1914 ± 25 e |
| OPO2 | |||||
| Chol | 1.08 ± 1.44a | 0.30 ± 0.05a | 0.27 ± 0.08a | 0.34 ± 0.04a | 0.30 ± 0.04a |
| Camp | 2.99 ± 0.03a | 2.98 ± 0.00a | 2.94 ± 0.00a | 2.98 ± 0.00a | 2.98 ± 0.00a |
| Stigm | 1.31 ± 0.08b | 1.28 ± 0.02ab | 1.24 ± 0.05ab | 1.22 ± 0.00a | 1.21 ± 0.02a |
| Δ7-Camp | 0.47 ± 0.04a | 0.48 ± 0.02a | 0.45 ± 0.01a | 0.43 ± 0.02a | 0.43 ± 0.03a |
| Cler | 0.02 ± 0.01a | 0.03 ± 0.03a | 0.04 ± 0.01a | 0.05 ±0.01a | 0.05 ± 0.01a |
| β-Sito | 86.16 ± 0.04a | 87.56 ± 0.05a | 87.67 ± 0.07a | 87.87 ±0.09a | 87.78 ± 0.09a |
| Sitos | 2.89 ± 1.36b | 2.66 ± 0.17a | 2.89 ± 0.31ab | 3.08 ± 0.30b | 3.03 ± 0.20b |
| Δ5-Aven | 3.31 ± 0.08a | 3.08 ± 0.13a | 2.98 ± 0.15a | 2.82± 0.08a | 2.80 ± 0.07b |
| Δ5,24-Sti | 1.05 ±0.04b | 0.93 ± 0.23ab | 0.95 ± 0.28ab | 0.79 ± 0.28a | 0.90 ± 0.06ab |
| Δ7-Stigmn | 0.36 ± 0.04b | 0.44 ± 0.03b | 0.34 ± 0.07ab | 0.29 ± 0.06a | 0.32 ± 0.09ab |
| Δ7-Aven | 0.27 ± 0.02a | 0.27 ± 0.08a | 0.24 ± 0.04a | 0.14 ± 0.02a | 0.19 ± 0.07a |
| Total (mg/kg) | 3021 ± 23a | 2892 ± 48b | 2643 ± 17c | 2416 ± 21 d | 2235 ± 23 e |
| OPO3 | |||||
| Chol | 0.23 ± 0.01a | 0.21 ± 0.04a | 0.27 ± 0.02a | 0.28 ± 0.09a | 0.21 ± 0.03a |
| Camp | 2.92 ± 0.04a | 2.92 ± 0.04a | 2.94 ± 0.15a | 2.93 ± 0.11a | 2.88 ± 0.10a |
| Stigm | 1.42 ± 0.03b | 1.36 ± 0.01ab | 1.32 ± 0.05ab | 1.34 ± 0.05ab | 1.32 ± 0.03a |
| Δ7-Camp | 0.81 ± 0.04a | 0.83 ± 0.05a | 0.79 ± 0.05a | 0.77 ± 0.05a | 0.71 ± 0.00a |
| Cler | 0.01 ± 0.03a | 0.01 ± 0.02a | 0.02 ± 0.05a | 0.03 ± 0.08a | 0.03 ± 0.05a |
| β-Sito | 87.11 ± 0.31a | 87.77 ± 0.19a | 87.43 ± 0.59a | 87.90 ± 0.38a | 88.11 ± 0.38a |
| Sitos | 2.92 ± 0.04a | 2.58 ± 0.10a | 3.04 ± 0.81a | 2.97 ± 0.55a | 3.18 ± 0.60a |
| Δ5-Aven | 1.82 ± 0.05a | 1.74 ± 0.08a | 1.76 ± 0.27a | 1.60 ± 0.23a | 1.54 ± 0.25a |
| Δ5,24-Sti | 2.10 ± 0.05c | 1.92 ± 0.11bc | 1.77 ± 0.12abc | 1.62 ± 0.17ab | 1.43 ± 0.15a |
| Δ7-Stigmn | 0.46 ± 0.12a | 0.40 ± 0.08a | 0.39 ± 0.15a | 0.33 ± 0.04a | 0.36 ± 0.12a |
| Δ7-Aven | 0.14 ± 0.07a | 0.20 ± 0.03a | 0.20 ± 0.06a | 0.18 ± 0.04a | 0.23 ± 0.13a |
| Total (mg/kg) | 3438 ± 17a | 3252 ± 25b | 3084 ± 56c | 2789 ± 36 d | 2606 ± 13 e |
| Tocopherols (mg/kg) | Squalene (mg/kg) | Aliphatic Alcohols (mg/kg) | Erythrodiol (mg/kg) | Oleanolic Acid (mg/kg) | |
|---|---|---|---|---|---|
| OPO1 | |||||
| 0 h | 496 ± 13a | 1062 ± 32a | 1875 ± 56a | 616 ± 13a | 210 ± 10a |
| 2 h | 215 ± 13b | 525 ± 21b | n.a. | n.a. | n.a. |
| 5 h | 45 ± 3c | 407 ± 20c | 2066 ± 152a | 552 ± 73ab | 142 ± 8b |
| 8 h | 9 ± 1d | 342 ± 10d | n.a. | n.a. | n.a. |
| 10 h | 10 ± 5d | 302 ± 10de | 2177 ± 465a | 532 ± 14ab | 119 ± 9c |
| 15 h | 0 | 266 ± 13e | 1895 ± 111a | 505 ± 3b | n.a. |
| 20 h | 0 | 166 ± 24f | 1938 ± 16a | 487 ± 18b | 105 ± 12c |
| OPO2 | |||||
| 0 h | 344 ± 6a | 3699 ± 58a | 1815 ± 5a | 585 ± 39a | 425 ± 45a |
| 2 h | 167 ± 67b | 3363 ± 21b | 1788 ± 19ab | 594 ± 16a | n.a. |
| 5 h | 23 ± 0c | n.a. | n.a. | n.a. | 380 ± 43a |
| 8 h | 25 ± 1c | 2172 ± 43c | 1788 ± 32ab | 525 ± 8b | n.a. |
| 10 h | 26 ± 1c | n.a. | n.a. | n.a. | 287 ± 5b |
| 15 h | 29 ± 2c | 1412 ± 32d | 1766 ± 30ab | 467 ± 15c | n.a. |
| 20 h | 23 ± 2c | 1083 ± 23e | 1738 ± 20b | 426 ± 1c | 271 ± 8b |
| OPO3 | |||||
| 0 h | 310 ± 4a | 491 ± 28a | 2136 ± 33a | 660 ± 18a | 358 ± 52a |
| 2 h | 283 ± 25a | 387 ± 13b | 2084 ± 22ab | 646 ± 10ab | n.a. |
| 5 h | 219 ± 20b | n.a. | n.a. | n.a. | 189 ± 9b |
| 8 h | 47 ± 13c | 267 ± 18c | 2104 ± 47ab | 608 ± 25b | n.a. |
| 10 h | 43 ± 4c | n.a. | n.a. | n.a. | 175 ± 20c |
| 15 h | 40 ± 4c | 166 ± 13d | 2032 ± 30ab | 536 ± 12c | n.a. |
| 20 h | 37 ± 5c | 116 ± 13e | 1989 ± 78b | 499 ± 4c | 102 ± 28c |
| Polar Compounds (wt%) | Total | Oligomers | Dimers | Oxidized Triacylglycerols | Diacyl Glycerols | Monoacyl Glycerols | Free Fatty Acids |
|---|---|---|---|---|---|---|---|
| Initial OPO | 7.6 ± 0.4 | nd | 0.6 ± 0.1 | 0.9 ± 0.1 | 5.4 ± 0.2 | 0.2 ± 0.0 | 0.5 ± 0.0 |
| French fries | |||||||
| Initial | 28.3 ± 02b | 6.9 ± 0.1b | 9.3 ± 0.1b | 8.6 ± 0.1b | 2.7 ± 0.1a | nd | 0.8 ± 0.0b |
| Fried | 14.2 ± 0.2a | 1.0 ± 0.1a | 3.6 ± 0.1a | 3.9 ± 0.4a | 4.9 ± 0.3b | 0.1 ± 0.0a | 0.6 ± 0.0a |
| Final OPO | 14.5 ± 0.3a | 0.9 ± 0.0a | 3.7 ± 0.1a | 3.9 ± 0.1a | 5.3 ± 0.2b | 0.2 ± 0.0b | 0.6 ± 0.0a |
| Croquettes | |||||||
| Initial | 8.1 ± 0.4a | 1.5 ± 0.2b | 0.8 ± 0.1a | 4.6 ± 0.2b | 1.2 ± 0.0a | nd | 1.1 ± 0.28c |
| Fried | 12.0 ± 2.0b | 0.8 ± 0.2a | 2.6 ± 0.6b | 4.5 ± 0.8b | 3.3 ± 0.4b | 0.1±0.01a | 0.75 ± 0.04b |
| Final OPO | 13.8 ± 0.9b | 0.6 ± 0.1a | 2.5 ± 0.3b | 2.6 ± 0.4a | 7.6 ± 0.3c | 0.16±0.05a | 0.47 ± 0.12a |
| Nuggets | |||||||
| Initial | 8.6 ± 1.3a | nd | 2.6 ± 0.6a | 3.3 ± 0.4a | 1.7 ± 0.4a | nd | 1 ± 0.1b |
| Fried | 10.3 ± 1.44b | 0.4 ± 0.08a | 1.8 ± 0.32a | 2.3 ± 0.29a | 4 ± 0.89b | 0.7 ± 0.19b | 1 ± 0.32b |
| Final OPO | 13.4 ± 2.2b | 0.5 ± 0.2a | 2.2 ± 0.6a | 2.3 ± 0.8a | 7.9 ± 0.6c | 0.1 ± 0.01a | 0.3 ± 0.1a |
| OPO | French Fries | Croquettes | Nuggets | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty Acid (%) | Initial | Initial | Fried | Final OPO | Initial | Fried | Final OPO | Initial | Fried | Final OPO |
| C16:0 | 12.02 ± 0.01 | 11.55 ± 0.03a | 11.99 ± 0.02b | 12.19 ± 0.02c | 14.44 ± 0.02c | 12.83 ± 0.14b | 12.19 ± 0.02a | 16.9 ± 0.02b | 17.54 ± 0.1b | 12.21 ± 0.09a |
| C16:1 | 0.87 ± 0.00 | 0.13 ± 0.01a | 0.84 ± 0.01b | 0.89 ± 0.00c | 1.55 ± 0.00c | 1.08 ± 0.03b | 0.9 ± 0.01a | 3.73 ± 0.02b | 2.16 ± 1.85b | 0.9 ± 0.02a |
| C17:0 | 0.09 ± 0.00 | nd | 0.09 ± 0.00a | 0.09 ± 0.00a | 0.16 ± 0.00c | 0.12 ± 0.01b | 0.10 ± 0.01a | 0.09 ± 0.01a | 0.11 ± 0.01a | 0.09 ± 0.01a |
| C17:1 | 0.15 ± 0.01 | nd | 0.14 ± 0.01a | 0.15 ± 0.00a | 0.14 ± 0.00ab | 0.13 ± 0.01a | 0.15 ± 0.01b | 0.07 ± 0.01a | 0.13 ± 0.01b | 0.15 ± 0.01b |
| C18:0 | 2.94 ± 0.01 | 3.43 ± 0.01b | 3.04 ± 0.00a | 3.05 ± 0.01a | 6.31 ± 0.01c | 4.52 ± 0.17b | 3.05 ± 0.01a | 4.77 ± 0.01c | 4.23 ± 0.08b | 3.02 ± 0.09a |
| C18:1 | 70.41 ± 0.07 | 42.52 ± 0.06a | 67.79 ± 0.1b | 70.13 ± 0.05c | 38.42 ± 0.02a | 54.1 ± 1.15b | 70.3 ± 0.02c | 43.73 ± 0.06a | 57.44 ± 0.11b | 69.91 ± 0.29c |
| C18:2 | 11.47 ± 0.02 | 40.62 ± 0.09c | 13.43 ± 0.1b | 10.85 ± 0.09a | 35.25 ± 0.07c | 23.95 ± 0.83b | 10.69 ± 0.07a | 27.18 ± 0.01c | 13.81 ± 0.27b | 11.12 ± 0.3a |
| C18:3 | 0.72 ± 0.01 | 0.24 ± 0.02a | 0.67 ± 0.01c | 0.60 ± 0.01b | 0.45 ± 0.01a | 0.54 ± 0.02b | 0.61 ± 0.01c | 0.60 ± 0.01a | 0.76 ± 0.01b | 0.60 ± 0.02a |
| C20:0 | 0.46 ± 0.01 | 0.24 ± 0.01a | 0.47 ± 0.01b | 0.50 ± 0.00c | 0.22 ± 0.00a | 0.37 ± 0.01b | 0.50 ± 0.01c | 0.23 ± 0.00a | 0.37 ± 0.00b | 0.50 ± 0.00c |
| C20:1 | 0.31 ± 0.00 | 0.17 ± 0.00a | 0.35 ± 0.00b | 0.36 ± 0.01b | 0.49 ± 0.00b | 0.39 ± 0.01a | 0.37 ± 0.01a | 0.34 ± 0.01a | 0.39 ± 0.01c | 0.37 ± 0.00b |
| C22:0 | nd | 0.54 ± 0.03c | 0.22 ± 0.00b | 0.20 ± 0.00a | 0.36 ± 0.00c | 0.01 ± 0.01a | 0.19 ± 0.01b | 0.04 ± 0.00a | 0.03 ± 0.00a | 0.20 ± 0.00b |
| C24:0 | nd | 0.19 ± 0.01b | 0.09 ± 0.01a | 0.08 ± 0.00a | 0.12 ± 0.00c | 0.11 ± 0b | 0.08 ± 0.01a | 0.12 ± 0.01c | 0.06 ± 0.00a | 0.08 ± 0.01b |
| Others | 0.37 ± 0.09 | 0.22 ± 0.01a | 0.59 ± 0.02b | 0.63 ± 0.04b | 1.8 ± 0.06c | 1.57 ± 0.03b | 0.57 ± 0.06a | 1.84 ± 0.12b | 2.66 ± 1.95b | 0.57 ± 0.06a |
| tC18:1 | 0.21 ± 0.00 | nd | 0.24 ± 0.00a | 0.24 ± 0.00a | 0.23 ± 0.01a | 0.22 ± 0.01a | 0.26 ± 0.01b | 0.20 ± 0.00a | 0.27 ± 0.01b | 0.27 ± 0.01b |
| tC18:2 | nd | 0.14 ± 0.01b | 0.04 ± 0.00a | 0.03 ± 0.01a | 0.09 ± 0.00b | 0.08 ± 0.01b | 0.03 ± 0.00a | 0.02 ± 0.01a | 0.05 ± 0.01b | 0.04 ± 0.00b |
| ∑trans | 0.21 ± 0.0 b | 0.14 ± 0.01a | 0.28 ± 0.01 b | 0.27 ± 0.01b | 0.32 ± 0.01b | 0.30 ± 0.01ab | 0.29 ± 0.01a | 0.32 ± 0.01a | 0.32 ± 0.01a | 0.31 ± 0.01a |
| Sterols | OPO | French Fries | Croquettes | Nuggets | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (% on Total) | Initial | Initial | Fried | Final OPO | Initial | Fried | Final OPO | Initial | Fried | Final OPO |
| Cholest | 0.34 ± 0.02 | 0.59 ± 0.06a | 0.40 ± 0.20a | 0.55 ± 0.20a | 36.95 ± 0.66c | 21.86 ± 1.93b | 0.50 ± 0.07a | 61.20 ± 1.40c | 55.53 ± 0.89b | 0.54 ± 0.23a |
| Brassicast | 0.11 ± 0.01 | 0.19 ± 0.08 | nd | nd | nd | 0.01 ± 0.05a | 0.52 ± 0.90b | nd | 0.16 ± 0.02a | 0.07 ± 0.12a |
| Campest | 3.30 ± 0.20 | 7.47 ± 0.07b | 3.59 ± 0.60a | 3.64 ± 0.60a | 6.85 ± 0.01a | 5.80 ± 0.05a | 4.45 ± 2.09a | 4.17 ± 0.14c | 2.48 ± 0.01a | 3.14 ± 0.05b |
| Campest | 0.35 ± 0.02 | 0.94 ± 0.12 | nd | nd | 1.16 ± 0.02 | 0.71 ± 0.00 | 0.19 ± 0.02 | 0.52 ± 0.02b | 0.39 ± 0.02a | nd |
| Stigmast | 1.55 ± 0.08 | 7.48 ± 0.16b | 1.77 ± 0.60a | 1.84 ± 0.60a | 4.24 ± 0.08c | 3.14 ± 0.02b | 1.08 ± 0.08a | 2.57 ± 0.09c | 0.58 ± 0.50a | 1.3 ± 0.20b |
| Δ7-Campest | 0.87 ± 0.04 | 2.08 ± 0.12b | 0.25 ± 0.2a | 0.25 ± 0.20a | 0.97 ± 0.13b | 0.80 ± 0.07b | 0.10 ± 0.09a | 0.81 ± 0.03b | 0.17 ± 0.01a | nd |
| Δ5,23-Stigmastadienol | 1.20 ± 0.06 | 1.28 ± 0.24a | 1.28 ± 0.01a | 1.30 ± 0.10a | 0.37 ± 0.04a | 0.77 ± 0.06b | 1.12 ± 0.06c | 0.27 ± 0.02a | 0.42 ± 0.11b | 1.16 ± 0.04c |
| Clerost | nd | 2.83 ± 1.26 | nd | nd | 0.29 ± 0.41b | nd | 0.03 ± 0.06a | 0.03 ± 0.03a | 0.16 ± 0.28a | nd |
| β-Sitost | 85.46 ± 4.30 | 49.88 ± 1.91a | 84.55 ± 2.80b | 84.51 ± 1.20b | 36.60 ±0.19a | 56.84 ± 1.91b | 85.72 ± 2.68c | 21.33 ± 0.77a | 36.43 ± 0.54b | 87.12 ± 0.75c |
| Sitost | 2.11 ± 0.10 | 2.39 ± 0.24b | 2.05 ± 0.20ab | 1.91 ± 0.10a | nd | nd | nd | 1.36 ± 0.03a | 1.00 ± 0.84a | 3.00 ± 0.63b |
| Δ5-Avenast | 1.66 ± 0.08 | 4.18 ± 0.25b | 2.48 ± 1.00a | 2.33 ± 0.70a | 1.54 ± 0.38a | 2.04 ± 0.15ab | 2.44 ± 0.04b | 1.16 ± 0.11b | 0.82 ± 0.04a | 2.44 ± 0.44c |
| Δ5,24-Stigmastadienol | 2.07 ± 0.10 | 2.05 ± 0.56a | 1.68 ± 0.30a | 1.79 ± 0.50a | 0.49 ± 0.11a | 1.71 ± 0.07b | 1.58 ± 0.02b | 0.47 ± 0.01a | 0.59 ± 0.05b | 1.59 ± 0.07c |
| Δ7-Stigmastenol | 0.62 ± 0.03 | 15.19 ± 0.71b | 1.56 ± 0.90a | 1.52 ± 0.90a | 5.91 ± 0.05c | 1.08 ± 0.06a | 1.53 ± 0.07b | 4.26 ± 0.16c | 0.79 ± 0.06a | 1.57 ± 0.05b |
| Δ7-Avenasterol | 0.30 ± 0.02 | 3.09 ± 0.27b | 0.40 ± 0.30a | 0.36 ± 0.30a | 2.04 ± 0.08b | 3.69 ± 0.09c | 0.45 ± 0.05a | 1.68 ± 0.13b | 0.31 ± 0.02a | 0.39 ± 0.33a |
| Others | nd | nd | nd | nd | 0.27 ± 0.04a | 1.46 ± 0.05b | 0.27 ± 0.03a | 0.19 ± 0.01a | 0.97 ± 0.84a | 3.13 ± 0.63b |
| Total Sterols (mg/kg) | 2515 ± 126 | 3963 ± 100b | 2707 ± 111a | 2703 ± 87a | 3494 ± 46b | 2282 ± 460a | 2146 ± 174a | 3509 ± 34c | 3036 ± 76b | 2498 ± 77a |
| OPO | French Fries | Croquettes | Nuggets | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Initial | Fried | Final OPO | Initial | Fried | Final OPO | Initial | Fried | Final OPO | |
| Tocopherols (mg/kg) | 496 ± 13 | 194 ± 2b | 102 ± 24a | 93 ± 22a | nd | 22 ± 30a | 115 ± 27b | 187 ± 10a | 82 ± 10a | 117 ± 81a |
| Squalene (mg/kg) | 1062 ± 32 | 66 ± 3a | 715 ± 21b | 804 ± 18c | 17 ± 2a | 362 ± 15b | 596 ± 21c | 58 ± 5a | 358 ± 62b | 601 ± 44c |
| Triterpenic Alcohols (mg/kg) | 708 ± 45 | nd | 638 ± 29a | 664 ± 7a | nd | 320 ± 11a | 609 ± 33b | nd | 281 ± 16a | 532 ± 23b |
| Erythrodiol | 636 ± 40 | nd | 574 ± 27a | 596 ± 5a | nd | 294 ± 20a | 562 ± 23b | nd | 259 ± 26a | 574 ± 33b |
| Uvaol | 72 ± 5 | nd | 65 ± 2a | 68 ± 2a | nd | 26 ± 4a | 47 ± 5b | nd | 22 ± 3a | 58 ± 4b |
| Triterpenic Acids (mg/kg) | 221 ± 11 | 33 ±2a | 175 ± 8c | 198 ± 3b | 31 ± 0a | 96 ± 12b | 192 ± 11c | 4 ± 1a | 97 ± 6b | 180 ± 6c |
| Oleanolic acid | 201 ± 10 | 31 ± 2a | 173 ± 8c | 196 ± 3b | 15 ± 0a | 93 ± 12b | 188 ± 11c | 4 ± 1a | 94 ± 6b | 174 ± 11c |
| Ursolic acid | 15 ± 1 | 2.0 ± 0.1a | 2.0 ± 0.1a | 2.0 ± 0.1a | 15 ± 3c | 2.0 ± 0.1a | 4.0 ± 0.1b | nd | 2.0 ± 0.1a | 4.0 ± 0.1b |
| Maslinic acid | 5.0 ± 0.2 | nd | nd | nd | nd | 1.0 ± 0.1 | nd | nd | 1.0 ± 0.1a | 2.0 ± 0.1b |
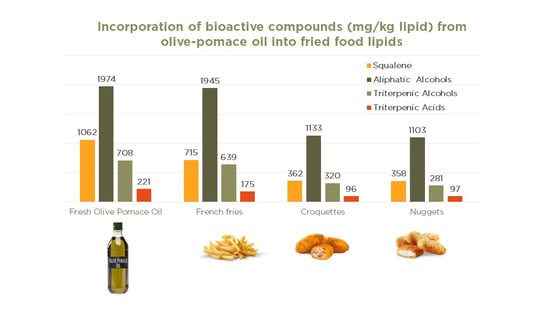
| Aliphatic Alcohols (mg/kg) | 1974 ± 56 | nd | 1945 ± 103a | 1839 ± 211a | nd | 1133 ± 63a | 1906 ± 16b | nd | 1103 ± 22a | 1960 ± 49b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Méndez, M.-V.; Márquez-Ruiz, G.; Holgado, F.; Velasco, J. Stability of Bioactive Compounds in Olive-Pomace Oil at Frying Temperature and Incorporation into Fried Foods. Foods 2021, 10, 2906. https://doi.org/10.3390/foods10122906
Ruiz-Méndez M-V, Márquez-Ruiz G, Holgado F, Velasco J. Stability of Bioactive Compounds in Olive-Pomace Oil at Frying Temperature and Incorporation into Fried Foods. Foods. 2021; 10(12):2906. https://doi.org/10.3390/foods10122906
Chicago/Turabian StyleRuiz-Méndez, María-Victoria, Gloria Márquez-Ruiz, Francisca Holgado, and Joaquín Velasco. 2021. "Stability of Bioactive Compounds in Olive-Pomace Oil at Frying Temperature and Incorporation into Fried Foods" Foods 10, no. 12: 2906. https://doi.org/10.3390/foods10122906