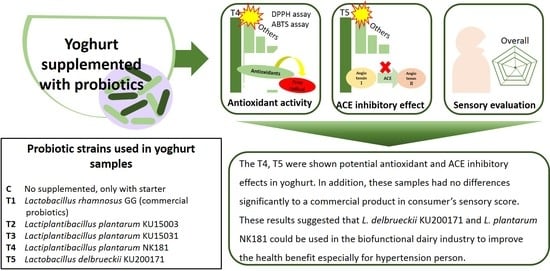
Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Conditions
2.2. Yogurt Sample Preparation
2.3. pH and LAB Viability of Yogurt
2.4. Preparation of Water-Soluble Extracts
2.5. Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Determination of ACE Inhibitory Activity
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. pH and Viability of LAB of Yogurt Samples
3.2. Antioxidant Activities of Yogurts
3.3. ACE Inhibition Activities of Yogurt
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarkar, S. Effect of probiotics on biotechnological characteristics of yoghurt. Br. Food J. 2008, 110, 717–740. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; de Vrese, M. Probiotics, prebiotics, and synbiotics-approaching a definition. Am. J. Clin. Nutr. 2001, 73, 361–364. [Google Scholar] [CrossRef] [Green Version]
- Oelschlaeger, T.A. Mechanisms of probiotic actions–A review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Soccol, C.R.; de Souza Vandenberghe, L.P.; Spier, M.R.; Medeiros, A.B.; Yamaguishi, C.T.; Lindner, J.D.D. The potential of probiotics: A review. Food Technol. Biotechnol. 2010, 48, 413–434. [Google Scholar]
- Lee, J.E.; Lee, N.K.; Paik, H.D. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef]
- Meltem, A.O.; Ayse, G. Lactobacillus fermentum strains from human breast milk with probiotic properties and cholesterol-lowering effects. Food Sci. Biotechnol. 2019, 28, 501–509. [Google Scholar]
- Liu, D.M.; Guo, J.; Zeng, X.A.; Sun, D.W.; Brennan, C.S.; Zhou, Q.X.; Zhou, J.S. The probiotic role of Lactobacillus plantarum in reducing risks associated with cardiovascular disease. Int. J. Food Sci. Technol. 2017, 52, 127–136. [Google Scholar] [CrossRef]
- Jeong, C.H.; Ryu, H.; Zhang, T.; Lee, C.H.; Seo, H.G.; Han, S.G. Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci. Biotechnol. 2018, 27, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.L.; Silva, R.; Cappato, L.P.; Almada, C.N.; Garcia, R.K.; Silva, M.C. Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological, physical–chemical and metabolic activity analyses. Food Res. Int. 2015, 77, 627–635. [Google Scholar] [CrossRef]
- Farvin, K.S.; Baron, C.P.; Nielsen, N.S.; Jacobsen, C. Antioxidant activity of yoghurt peptides: Part 1-in vitro assays and evaluation in ω-3 enriched milk. Food Chem. 2010, 123, 1081–1089. [Google Scholar] [CrossRef]
- Kariyawasam, K.M.G.M.M.; Lee, N.K.; Paik, H.D. Synbiotic yoghurt supplemented with novel probiotic Lactobacillus brevis KU200019 and fructooligosaccharides. Food Biosci. 2021, 39, 100835. [Google Scholar] [CrossRef]
- Arief, I.I.; Taufik, E. Quality and antioxidant activity of yogurt supplemented with roselle during cold storage. Media Peternak. 2016, 39, 82–89. [Google Scholar]
- Ovando, C.A.; Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional properties and health benefits of bioactive peptides derived from spirulina: A review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.M.B.; Fatemizadeh, S.S.; Tavakoli, M. Release of proteolysis products with ACE-inhibitory and antioxidant activities in probiotic yogurt containing different levels of fat and prebiotics. Int. J. Pept. Res. Ther. 2019, 25, 367–377. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Effect of probiotics on antioxidant and antimutagenic activities of crude peptide extract from yogurt. Food Chem. 2014, 156, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Shah, N.P. Influence of probiotic Lactobacillus acidophilus and L. helveticus on proteolysis, organic acid profiles, and ACE-inhibitory activity of cheddar cheeses ripened at 4, 8, and 12 °C. J. Food Sci. 2008, 73, 111–120. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, H.W.; Chang, H.I.; Yun, C.W.; Kim, S.W.; Kang, C.W.; Paik, H.D. Probiotic properties of Lactobacillus plantarum NK181 isolated from jeotgal, a Korean fermented food. Food Sci. Biotechnol. 2006, 15, 227–231. [Google Scholar]
- Kariyawasam, K.M.G.M.M.; Jeewanthi, R.K.C.; Lee, N.K.; Paik, H.D. Characterization of cottage cheese using Weissella cibaria D30: Physicochemical, antioxidant, and antilisterial properties. J. Dairy Sci. 2019, 102, 3887–3893. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Lee, J.E.; Lim, S.M.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin converting enzyme carboxyalkanoyl and mercaptoalkanoyl amino acid. Biochemistry 1977, 16, 54–84. [Google Scholar] [CrossRef]
- ISO 5496: 2006; Sensory Analysis—Methodology—Initiation and Training of Assessors in the Detection and Recognition of Odours; ISO: Geneva, Switzerland, 1992.
- Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Review Article: Sensory characteristics of probiotic cheese. Compr. Rev. Food Sci. Food Saf. 2012, 11, 437–452. [Google Scholar] [CrossRef]
- Szabo, M.; Iditoiu, C.; Chambre, D.; Lupea, A. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Nicholas, J.M.; Catherine, A.R.E. Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar]
- Dziuba, M.; Darewicz, M. Food proteins as precursors of bioactive peptides classification into families. Food Sci. Technol. Int. 2007, 13, 393–404. [Google Scholar] [CrossRef]
- Tang, X.; He, Z.; Dait, Y.; Xiong, Y.L.; Xie, M.; Chen, J. Peptide fractionation and free radical scavenging activity of zein. J. Agric. Food Chem. 2010, 58, 587–593. [Google Scholar] [CrossRef]
- Kudoh, Y.; Matsuda, S.; Igoshi, K.; Oki, T. Antioxidative peptide from milk fermented with Lactobacillus delbrueckii ssp. bulgaricus IFO13953. J. Jpn. Soc. Food Sci. 2001, 48, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin converting enzyme of rabbit lung. Biochem. Pharm. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Cavalheiro, G.F.; Baptista, D.P.; Galli, B.D.; Negrão, F.; Eberlin, M.N.; Gigante, M.L. High protein yogurt with addition of Lactobacillus helveticus: Peptide profile and angiotensin-converting enzyme ACE-inhibitory activity. Food Chem. 2020, 333, 127482. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Tsai, J.S.; Sun Pan, B. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics from metchnikoff to bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Habibi, N.M.B.; Tavakoli, M.; Fatemizadeh, S.S. Investigation on the effect of fat content and prebiotic compound on proteolysis rate, ACE-inhibitory and antioxidant activity of probiotic yogurt. J. Appl. Microbiol. Food Ind. 2017, 3, 16–29. [Google Scholar]
- Erkaya-Kotan, T. In vitro angiotensin converting enzyme (ACE)-inhibitory and antioxidant activity of probiotic yogurt incorporated with orange fibre during storage. J. Food Sci. Technol. 2020, 57, 2343–2353. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Probiotic strains as starter cultures improve angiotensin-converting enzyme inhibitory activity in soy yogurt. J. Food Sci. 2005, 70, 375–381. [Google Scholar] [CrossRef]
- Cheon, M.J.; Lim, S.M.; Lee, N.K.; Paik, H.D. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef] [Green Version]




| Sensory Evaluation | Samples | |||
|---|---|---|---|---|
| C | T1 | T4 | T5 | |
| Color | 5.71 ± 1.35 | 5.61 ± 1.33 | 5.39 ± 1.31 | 5.48 ± 1.23 |
| Taste | 4.10 ± 1.33 | 4.19 ± 1.35 | 4.58 ± 1.31 | 4.55 ± 1.39 |
| Texture | 4.80 ± 1.40 | 5.19 ± 1.11 | 5.29 ± 1.30 | 5.06 ± 1.50 |
| Flavor | 4.90 ± 1.04 | 5.39 ± 0.99 | 5.42 ± 0.99 | 5.39 ± 0.88 |
| Overall | 4.16 ± 1.37 | 4.58 ± 1.20 | 4.61 ± 1.36 | 4.74 ± 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-D.; Lee, H.-S.; Kim, K.-T.; Paik, H.-D. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation. Foods 2021, 10, 2324. https://doi.org/10.3390/foods10102324
Kim E-D, Lee H-S, Kim K-T, Paik H-D. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation. Foods. 2021; 10(10):2324. https://doi.org/10.3390/foods10102324
Chicago/Turabian StyleKim, Eun-Deok, Hyun-Sook Lee, Kee-Tae Kim, and Hyun-Dong Paik. 2021. "Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activities of Yogurt Supplemented with Lactiplantibacillus plantarum NK181 and Lactobacillus delbrueckii KU200171 and Sensory Evaluation" Foods 10, no. 10: 2324. https://doi.org/10.3390/foods10102324