The Effect of Oral Care Foams and a Spray on Salivary pH Changes after Exposure to Acidic Beverages in Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
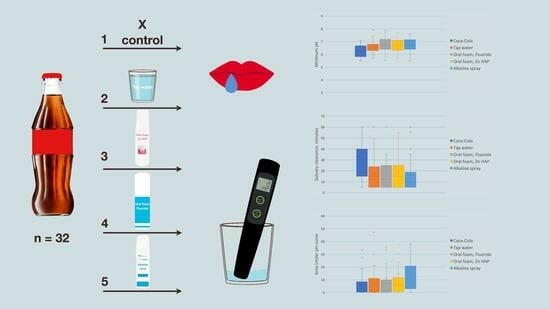
2.2. Study Design
2.3. Sampling Criteria
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.3.3. Elimination Criteria
2.4. Randomization
2.5. Interventions and Outcomes
- WF and BF were squeezed (2 pumps of the foams) into the mouth, swished for 30 s, and spat out without rinsing.
- BS was sprayed in the mouth (2 sprays) and left for 30 s; could be swallowed.
- A sip of tap water was swished in the mouth for 30 s and spat out.
2.6. Statistical Analysis
2.7. Data Management
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.S.; Freedman, N.D.; Kamangar, F.; Dawsey, S.M.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Tea, Coffee, Carbonated Soft Drinks and Upper Gastrointestinal Tract Cancer Risk in a Large United States Prospective Cohort Study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.J.; Popkin, B.M. Changes in Beverage Intake between 1977 and 2001. Am. J. Prev. Med. 2004, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; McKee, M.; Galea, G.; Stuckler, D. Relationship of Soft Drink Consumption to Global Overweight, Obesity, and Diabetes: A Cross-National Analysis of 75 Countries. Am. J. Public Health 2013, 103, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Hawkes, C. Sweetening of the Global Diet, Particularly Beverages: Patterns, Trends, and Policy Responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Lasater, G.; Piernas, C.; Popkin, B.M. Beverage Patterns and Trends among School-Aged Children in the US, 1989–2008. Nutr. J. 2011, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tuan, H.; Na, X.; Yang, H.; Yang, Y.; Zhang, Y.; Xi, M.; Tan, Y.; Yang, C.; Zhang, J.; et al. The Association between Sugar-Sweetened Beverages and Male Pattern Hair Loss in Young Men. Nutrients 2023, 15, 214. [Google Scholar] [CrossRef]
- Terry-McElrath, Y.M.; O’Malley, P.M.; Johnston, L.D. Energy Drinks, Soft Drinks, and Substance Use among United States Secondary School Students. J. Addict. Med. 2014, 8, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Coday, M.; Garcia, D.O.; Li, X.; Mossavar-Rahmani, Y.; Naughton, M.J.; Lopez-Pentecost, M.; Saquib, N.; Shadyab, A.H.; et al. Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Liver Cancer and Chronic Liver Disease Mortality. JAMA 2023, 330, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Khanferyan, R.A.; Vybornaya, K.V.; Radzhabkadiev, R.M.; Evstratova, V.S.; Nalivayko, N.V.; Semin, V.B.; Galstyan, A.G. Frequency of Consumption of Sweet Carbonated Drinks by the Population of Different Age Groups of the Russian Federation. Vopr. Pitan. [Probl. Nutr.] 2017, 86, 55–58. [Google Scholar]
- Petrova, M.M.; Pronina, E.A.; Yaganova, S.S.; Anonen, P.Y.; Demakova, M.Y. Investigation of Sugar-Sweetened Beverages Consumption in Students of Krasnoyarsk State Medical University Named after V.F. Voino-Yasenetsky. Vopr. Pitan. [Probl. Nutr.] 2017, 86, 93–98. [Google Scholar]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Mancini, A.; Inchingolo, F.; Inchingolo, A.D.; Di Venere, D.; Dipalma, G.; et al. Damage from Carbonated Soft Drinks on Enamel: A Systematic Review. Nutrients 2023, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. Sugar-Sweetened Beverages and Cardiometabolic Health: An Update of the Evidence. Nutrients 2019, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Pan, N.; Xu, X.; Li, H.; Lin, L.; Chen, J.; Jin, C.; Pan, S.; Jing, J.; Li, X. The Association between Sugar-Sweetened Beverages and Milk Intake with Emotional and Behavioral Problems in Children with Autism Spectrum Disorder. Front. Nutr. 2022, 9, 927212. [Google Scholar] [CrossRef]
- Kashino, I.; Kochi, T.; Imamura, F.; Eguchi, M.; Kuwahara, K.; Nanri, A.; Kurotani, K.; Akter, S.; Hu, H.; Miki, T.; et al. Prospective Association of Soft Drink Consumption with Depressive Symptoms. Nutrition 2021, 81, 110860. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.J.; Waterhouse, B.; Aggarwal, V.R.; Bloor, K.; Doran, T. Effect of Sugar-Sweetened Beverages on Oral Health: A Systematic Review and Meta-Analysis. Eur. J. Public Health 2021, 31, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, H.; Romaniuk, P. Relationship between Consumption of Soft and Alcoholic Drinks and Oral Health Problems. Cent. Eur. J. Public Health 2020, 28, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Song, I.-S.; Han, K.; Ko, Y.; Park, Y.-G.; Ryu, J.-J.; Park, J.-B. Associations between the Consumption of Carbonated Beverages and Periodontal Disease. Medicine 2016, 95, e4253. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.M.; Vani, N.V.; Almutari, D.A.; Jafar, M.A.; Boreak, N. Analysis of Sugars and PH in Commercially Available Soft Drinks in Saudi Arabia with a Brief Review on Their Dental Implications. J. Int. Soc. Prev. Community Dent. 2016, 6, S192–S196. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A.; Pailahual, V.; Díaz-Garrido, N. Cariogenicity Induced by Commercial Carbonated Beverages in an Experimental Biofilm-Caries Model. Eur. J. Dent. 2018, 12, 27–35. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Gargani, A.; Parcianello, R.G.; Pezzato, L.; Bertolini, R.; Zuccon, A.; Stellini, E.; Ludovichetti, F.S. Protection against Dental Erosion and the Remineralization Capacity of Non-Fluoride Toothpaste, Fluoride Toothpaste and Fluoride Varnish. Appl. Sci. 2023, 13, 1849. [Google Scholar] [CrossRef]
- Ruiz, D.C.; Marqués Martínez, L.; García Miralles, E. Dental Erosion and Diet in Young Children and Adolescents: A Systematic Review. Appl. Sci. 2023, 13, 3519. [Google Scholar] [CrossRef]
- Vieira Pedrosa, B.R.; de Menezes, V.A. Prevalence of Erosive Tooth Wear and Related Risk Factors in Adolescents: An Integrative Review. J. Dent. Child (Chic) 2020, 87, 18–25. [Google Scholar] [PubMed]
- Salas, M.M.S.; Nascimento, G.G.; Huysmans, M.C.; Demarco, F.F. Estimated Prevalence of Erosive Tooth Wear in Permanent Teeth of Children and Adolescents: An Epidemiological Systematic Review and Meta-Regression Analysis. J. Dent. 2015, 43, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.M.S.; Nascimento, G.G.; Vargas-Ferreira, F.; Tarquinio, S.B.C.; Huysmans, M.C.D.N.J.M.; Demarco, F.F. Diet Influenced Tooth Erosion Prevalence in Children and Adolescents: Results of a Meta-Analysis and Meta-Regression. J. Dent. 2015, 43, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Rius-Bonet, O.; Roca-Obis, P.; Zamora-Olave, C.; Willaert, E.; Martinez-Gomis, J. Prevalence of Dental Attrition and Its Relationship with Dental Erosion and Salivary Function in Young Adults. Quintessence Int. 2023, 54, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Jaeggi, T. Chemical Factors. Monogr. Oral. Sci. 2006, 20, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.E.; Brandao, A.C.S.; Bícego-Pereira, E.C.; Del Bel Cury, A.A.; Cury, J.A.; Tenuta, L.M.A. Effect of PH and Titratable Acidity on Enamel and Dentine Erosion. Clin. Oral Investig. 2022, 26, 5867–5873. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.; Ionta, F.-Q.; Rebelato, R.; Jordão, M.-C.; Wang, L.; Magalhães, A.-C.; Honório, H.-M. The Effect of Aspartame and PH Changes on the Erosive Potential of Cola Drinks in Bovine Enamel: An in Vitro Study. J. Clin. Exp. Dent. 2018, 10, e933–e937. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.R.; Shahnawaz, K.; Kumari, P.D.; Chowdhury, A.; Gootveld, M.; Lynch, E. Highly Acidic PH Values of Carbonated Sweet Drinks, Fruit Juices, Mineral Waters and Unregulated Fluoride Levels in Oral Care Products and Drinks in India: A Public Health Concern. Perspect. Public Health 2019, 139, 186–194. [Google Scholar] [CrossRef]
- Yip, H.H.Y.; Wong, R.W.K.; Hägg, U. Complications of Orthodontic Treatment: Are Soft Drinks a Risk Factor? World J. Orthod. 2009, 10, 33–40. [Google Scholar]
- González-Aragón Pineda, Á.E.; Borges-Yáñez, S.A.; Irigoyen-Camacho, M.E.; Lussi, A. Relationship between Erosive Tooth Wear and Beverage Consumption among a Group of Schoolchildren in Mexico City. Clin. Oral Investig. 2019, 23, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Dugmore, C.R.; Rock, W.P. A Multifactorial Analysis of Factors Associated with Dental Erosion. Br. Dent. J. 2004, 196, 283–286; discussion 273. [Google Scholar] [CrossRef] [PubMed]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, V.I.; Pereira-Cenci, T.; Walboomers, X.F.; Loomans, B.A.C. Association between Salivary Characteristics and Tooth Wear: A Systematic Review and Meta-Analysis. J. Dent. 2023, 138, 104692. [Google Scholar] [CrossRef] [PubMed]
- Tenovuo, J.; Rekola, M. Some Effects of Sugar-Flavored Acid Beverages on the Biochemistry of Human Whole Saliva and Dental Plaque. Acta Odontol. Scand. 1977, 35, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Torres, G.C.; Klünder-Klünder, M.; Garduño-Espinosa, J.; Parra-Ortega, I.; Franco-Hernández, M.I.; Miranda-Lora, A.L. Effects of Carbonated Beverage Consumption on Oral PH and Bacterial Proliferation in Adolescents: A Randomized Crossover Clinical Trial. Life 2022, 12, 1776. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.A.; Fernandez De Preliasco, M.V. Salivary pH Changes during Soft Drinks Consumption in Children. Int. J. Paediatr. Dent. 2003, 13, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hans, R.; Thomas, S.; Garla, B.; Dagli, R.J.; Hans, M.K. Effect of Various Sugary Beverages on Salivary PH, Flow Rate, and Oral Clearance Rate amongst Adults. Scientifica 2016, 2016, 5027283. [Google Scholar] [CrossRef]
- Tenuta, L.M.A.; Fernández, C.E.; Brandão, A.C.S.; Cury, J.A. Titratable Acidity of Beverages Influences Salivary PH Recovery. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Alkasso, I.R.; Salih Al Qassar, S.S.; Taqa, G.A. Durability of Different Types of Mouthwashes on the Salivary Buffering System in Orthodontic Patients. Dent. 3000 2021, 9, 178–192. [Google Scholar] [CrossRef]
- Lussi, A.; Schaffner, M. Progression of and Risk Factors for Dental Erosion and Wedge-Shaped Defects over a 6-Year Period. Caries Res. 2000, 34, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M.J.; Procopio, A.; Attin, T.; Wegehaupt, F.J. Homemade Modification of Salad Dressings to Reduce Their Erosive Potential. Oral. Health Prev. Dent. 2021, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.A.; Curzon, M.E. A Comparison of Acidic Dietary Factors in Children with and without Dental Erosion. ASDC J. Dent. Child. 2000, 67, 160, 186–192. [Google Scholar] [PubMed]
- Thomas, E.; Tayab, T.; Rai, K.; Kumari, V. Effect of Chewing Paneer and Cheese on Salivary Acidogenicity: A Comparative Study. Int. J. Clin. Pediatr. Dent. 2012, 5, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Rios, D.; Honório, H.M.; Magalhães, A.C.; Delbem, A.C.B.; Machado, M.A.A.M.; Silva, S.M.B.; Buzalaf, M.A.R. Effect of Salivary Stimulation on Erosion of Human and Bovine Enamel Subjected or Not to Subsequent Abrasion: An in Situ/Ex Vivo Study. Caries Res. 2006, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]
- de Alencar, C.R.B.; Magalhães, A.C.; de Andrade Moreira Machado, M.A.; de Oliveira, T.M.; Honório, H.M.; Rios, D. In Situ Effect of a Commercial CPP-ACP Chewing Gum on the Human Enamel Initial Erosion. J. Dent. 2014, 42, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Weiss, K.; Becker, K.; Buchalla, W.; Wiegand, A. Impact of Modified Acidic Soft Drinks on Enamel Erosion. Oral Dis. 2005, 11, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.C.; Moraes, S.M.; Rios, D.; Buzalaf, M.A.R. Effect of Ion Supplementation of a Commercial Soft Drink on Tooth Enamel Erosion. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Jensdottir, T.; Bardow, A.; Holbrook, P. Properties and Modification of Soft Drinks in Relation to Their Erosive Potential in Vitro. J. Dent. 2005, 33, 569–575. [Google Scholar] [CrossRef]
- Bologa, E.; Stoleriu, S.; Nica, I.; Tărăboanță, I.; Georgescu, A.; Matei, R.I.; Andrian, S. The Effect of Three Desensitizing Toothpastes on Dentinal Tubules Occlusion and on Dentin Hardness. Biomedicines 2023, 11, 2464. [Google Scholar] [CrossRef]
- Lussi, A.; Buzalaf, M.A.R.; Duangthip, D.; Anttonen, V.; Ganss, C.; João-Souza, S.H.; Baumann, T.; Carvalho, T.S. The Use of Fluoride for the Prevention of Dental Erosion and Erosive Tooth Wear in Children and Adolescents. Eur. Arch. Paediatr. Dent. 2019, 20, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Tulumbaci, F.; Gungormus, M. In Vitro Remineralization of Primary Teeth with a Mineralization-Promoting Peptide Containing Dental Varnish. J. Appl. Oral Sci. 2020, 28, e20200259. [Google Scholar] [CrossRef] [PubMed]
- Imran, E.; Cooper, P.R.; Ratnayake, J.; Ekambaram, M.; Mei, M.L. Potential Beneficial Effects of Hydroxyapatite Nanoparticles on Caries Lesions In Vitro-A Review of the Literature. Dent. J. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Scribante, A.; Dermenaki Farahani, M.R.; Marino, G.; Matera, C.; Rodriguez Y Baena, R.; Lanteri, V.; Butera, A. Biomimetic Effect of Nano-Hydroxyapatite in Demineralized Enamel before Orthodontic Bonding of Brackets and Attachments: Visual, Adhesion Strength, and Hardness in In Vitro Tests. BioMed Res. Int. 2020, 2020, 6747498. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.D.; Pop, L.C.; Benea, H.R.C.; Tomoaia, G.; Racz, C.P.; Mocanu, A.; Dobrota, C.T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Tantbirojn, D.; Kymer-Davis, E.; Stewart, C.W.; Zhang, Y.H.; Versluis, A.; Garcia-Godoy, F. Neutralizing Salivary PH by Mouthwashes after an Acidic Challenge. J. Investig. Clin. Dent. 2017, 8, e12198. [Google Scholar] [CrossRef]
- Novozhilova, N.; Andreeva, E.; Polyakova, M.; Makeeva, I.; Sokhova, I.; Doroshina, V.; Zaytsev, A.; Babina, K. Antigingivitis, Desensitizing, and Antiplaque Effects of Alkaline Toothpastes: A Randomized Clinical Trial. Dent. J. 2023, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kanenaga, R.; Tanaka, Y.; Hotta, K.; Arakawa, S. The Neutralizing Effect of Mouth Rinsing with Alkaline Electrolyzed Water on Different Regions of the Oral Cavity Acidified by Acidic Beverages. J. Oral Sci. 2022, 64, 17–21. [Google Scholar] [CrossRef]
- Wiegand, A.; Müller, I.; Schnapp, J.D.; Werner, C.; Attin, T. Impact of Fluoride, Milk and Water Rinsing on Surface Rehardening of Acid Softened Enamel. An in Situ Study. Am. J. Dent. 2008, 21, 113–118. [Google Scholar]
- Belardinelli, P.A.; Morelatto, R.A.; Benavidez, T.E.; Baruzzi, A.M.; López de Blanc, S.A. Effect of Two Mouthwashes on Salivary Ph. Acta Odontol. Latinoam. 2014, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, M.G.; Polyakova, M.A.; Babina, K.S.; Novozhilova, N.E.; Margaryan, E.G.; Doroshina, V.Y.; Arzukanyan, A.V.; Makeeva, M.K. Qualitative and Quantitative Evaluation of the Efficiency of the Application of Foams with False Xerostomia. J. Int. Soc. Prev. Community Dent. 2019, 9, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Baez, R.J. (Eds.) Oral Health Surveys: Basic Methods, 5th ed.; WHO Press: Geneve, Switzerland, 2013; ISBN 9783642204784. [Google Scholar]
- Greene, J.G.; Vermillion, J.R. The Simplified Oral Hygiene Index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Periodontal indexes for and in practice. Tandlaegebladet 1976, 80, 149–152. [Google Scholar] [PubMed]
- Foglio-Bonda, P.L.; Brilli, K.; Pattarino, F.; Foglio-Bonda, A. Salivary Flow Rate and PH in Patients with Oral Pathologies. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 369–374. [Google Scholar] [PubMed]
- Bowen, W.H. The Stephan Curve Revisited. Odontology 2013, 101, 2–8. [Google Scholar] [CrossRef]
- Shahmoon, R.; Tamir, Y.; Beiderman, Y.; Agdarov, S.; Beiderman, Y.; Zalevsky, Z. Analysis of Swallowing in Infants and Adults Using Speckle Pattern Analysis. Sci. Rep. 2022, 12, 3847. [Google Scholar] [CrossRef] [PubMed]
- Cassiani, R.A.; Santos, C.M.; Parreira, L.C.; Dantas, R.O. The Relationship between the Oral and Pharyngeal Phases of Swallowing. Clinics 2011, 66, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Sales-Peres, S.H.; Magalhães, A.C.; de Andrade Moreira Machado, M.A.; Buzalaf, M.A.R. Evaluation of the Erosive Potential of Soft Drinks. Eur. J. Dent. 2007, 1, 10–13. [Google Scholar] [CrossRef]
- Mojaver, Y.N.; Javidi, N.; Manshaee, K. Influence of Soft Drink on Salivary PH. Chin. J. Dent. Res. 2008, 11, 52–55. [Google Scholar]
- Barac, R.; Gasic, J.; Trutic, N.; Sunaric, S.; Popovic, J.; Djekic, P.; Radenkovic, G.; Mitic, A. Erosive Effect of Different Soft Drinks on Enamel Surface in Vitro: Application of Stylus Profilometry. Med. Princ. Pract. 2015, 24, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. What Is the Critical PH and Why Does a Tooth Dissolve in Acid? J. Can. Dent. Assoc. 2003, 69, 722–724. [Google Scholar] [PubMed]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental Erosion—An Overview with Emphasis on Chemical and Histopathological Aspects. Caries Res. 2011, 45 (Suppl. S1), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Epple, M.; Enax, J.; Meyer, F. Prevention of Caries and Dental Erosion by Fluorides—A Critical Discussion Based on Physico-Chemical Data and Principles. Dent. J. 2022, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, P.; Chuenarrom, C. Association of Dental Enamel Loss with the PH and Titratable Acidity of Beverages. J. Dent. Sci. 2011, 6, 129–133. [Google Scholar] [CrossRef]
- Barbour, M.E.; Lussi, A. Erosion in Relation to Nutrition and the Environment. Monogr. Oral. Sci. 2014, 25, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-H.; Son, H.-H.; Yi, K.; Chang, J. Elemental Analysis of Caries-Affected Root Dentin and Artificially Demineralized Dentin. Restor. Dent. Endod. 2016, 41, 255. [Google Scholar] [CrossRef] [PubMed]
- Widodo, G.; Wilson, R.; Bartlett, D. Oral Clearance of an Acidic Drink in Patients with Erosive Tooth Wear Compared with That in Control Subjects. Int. J. Prosthodont. 2005, 18, 323–327. [Google Scholar] [PubMed]
- Banan, L.K.; Hegde, A.M. Plaque and Salivary PH Changes after Consumption of Fresh Fruit Juices. J. Clin. Pediatr. Dent. 2005, 30, 9–13. [Google Scholar] [CrossRef]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team 2015, 2, 15123. [Google Scholar] [CrossRef]
- Hara, A.T.; Zero, D.T. The Potential of Saliva in Protecting against Dental Erosion. Monogr. Oral. Sci. 2014, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Fenoll-Palomares, C.; Muñoz Montagud, J.V.; Sanchiz, V.; Herreros, B.; Hernández, V.; Mínguez, M.; Benages, A. Unstimulated Salivary Flow Rate, PH and Buffer Capacity of Saliva in Healthy Volunteers. Rev. Esp. Enfermedades Dig. 2004, 96, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Mokeem, L.S.; Willis, L.H.; Jack Windsor, L.; Blaine Cook, N.; Eckert, G.; Gregory, R.L. Combined Effects of Soft Drinks and Nicotine on Streptococcus Mutans Metabolic Activity and Biofilm Formation. J. Oral Sci. 2021, 63, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.K.; Lingström, P.; Birkhed, D. Effect of Soft Drinks on Proximal Plaque PH at Normal and Low Salivary Secretion Rates. Acta Odontol. Scand. 2007, 65, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hedge, R.; Dixit, U. Role of Plaque in the Clearance of Salivary Sucrose and Its Influence on Salivary Ph. J. Indian. Soc. Pedod. Prev. Dent. 2011, 29, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Shaw, L. Baby Fruit Juices and Tooth Erosion. Br. Dent. J. 1987, 162, 65–67. [Google Scholar] [CrossRef]
- Grenby, T.H.; Mistry, M.; Desai, T. Potential Dental Effects of Infants’ Fruit Drinks Studied in Vitro. Br. J. Nutr. 1990, 64, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; von Salis-Marincek, M.; Ganss, C.; Hellwig, E.; Cheaib, Z.; Jaeggi, T. Clinical Study Monitoring the PH on Tooth Surfaces in Patients with and without Erosion. Caries Res. 2012, 46, 507–512. [Google Scholar] [CrossRef]
- Kershaw, J.C.; Running, C.A. Conditioning of Human Salivary Flow Using a Visual Cue for Sour Candy. Arch. Oral. Biol. 2018, 92, 90–95. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; Bikker, F.J.; Nazmi, K.; de Graaf, K.; Laine, M.L.; Boesveldt, S. A Stepwise Approach Investigating Salivary Responses upon Multisensory Food Cues. Physiol. Behav. 2020, 226, 113116. [Google Scholar] [CrossRef]
- Fibryanto, E.; Widyastuti, W. Effect of Brushing the Teeth before and after Meals on Salivary PH: A Quasi-Experimental Study. J. Int. Oral Health 2022, 14, 163. [Google Scholar] [CrossRef]
- Setiawan, S.; Haroen, E.R.; Hadidjah, D. The Difference in Saliva PH before and after Brushing with Fluoride Containing Toothpaste and without Toothpaste. Padjadjaran J. Dent. 2008, 20, 139. [Google Scholar] [CrossRef]
- Al Asmari, D.; Khan, M. Evaluate Efficacy of Desensitizing Toothpaste Containing Zinc-Carbonate Hydroxyapatite Nanocrystals: Non-Comparative Eight-Week Clinical Study. J. Int. Soc. Prev. Community Dent. 2019, 9, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef]
- Polyakova, M.; Sokhova, I.; Doroshina, V.; Arakelyan, M.; Novozhilova, N.; Babina, K. The Effect of Toothpastes Containing Hydroxyapatite, Fluoroapatite, and Zn-Mg-Hydroxyapatite Nanocrystals on Dentin Hypersensitivity: A Randomized Clinical Trial. J. Int. Soc. Prev. Community Dent. 2022, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of Ingesting Yogurt Fermented with Lactobacillus Delbrueckii Ssp. Bulgaricus OLL1073R-1 on Influenza Virus-Bound Salivary IgA in Elderly Residents of Nursing Homes: A Randomized Controlled Trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Sanghvi, U.; Chhabra, T.; Sethuraman, R. Effect of Probiotics on the Amount and PH of Saliva in Edentulous Patients: A Prospective Study. J. Indian Prosthodont. Soc. 2018, 18, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; López-López, A.; Nicolescu, T.; Perez-Vilaplana, S.; Boix-Amorós, A.; Dzidic, M.; Garcia, S.; Artacho, A.; Llena, C.; Mira, A. Topic Application of the Probiotic Streptococcus Dentisani Improves Clinical and Microbiological Parameters Associated With Oral Health. Front. Cell Infect. Microbiol. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Salikhova, D.; Doroshina, V.; Makeeva, I.; Zaytsev, A.; Uvarichev, M.; Polyakova, M.; Novozhilova, N. Antigingivitis and Antiplaque Effects of Oral Probiotic Containing the Streptococcus Salivarius M18 Strain: A Randomized Clinical Trial. Nutrients 2023, 15, 3882. [Google Scholar] [CrossRef]
- Kuru, B.E.; Laleman, I.; Yalnızoğlu, T.; Kuru, L.; Teughels, W. The Influence of a Bifidobacterium Animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1115–1123. [Google Scholar] [CrossRef]
- Schlagenhauf, U.; Rehder, J.; Gelbrich, G.; Jockel-Schneider, Y. Consumption of Lactobacillus Reuteri-Containing Lozenges Improves Periodontal Health in Navy Sailors at Sea: A Randomized Controlled Trial. J. Periodontol. 2020, 91, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Hambire, C.; Hambire, U. Evaluation of Effect of Consumption of Probiotics on the Gingival and Periodontal Health Status in Children Undergoing Chemotherapy. Indian J. Cancer 2023, 60, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Babina, K.; Salikhova, D.; Polyakova, M.; Svitich, O.; Samoylikov, R.; Ahmad El-Abed, S.; Zaytsev, A.; Novozhilova, N. The Effect of Oral Probiotics (Streptococcus Salivarius K12) on the Salivary Level of Secretory Immunoglobulin A, Salivation Rate, and Oral Biofilm: A Pilot Randomized Clinical Trial. Nutrients 2022, 14, 1124. [Google Scholar] [CrossRef] [PubMed]



| Short Name | Solution | Active Ingredient | pH | Titratable Acidity |
|---|---|---|---|---|
| Tap water | Tap water | Not applicable | pH = 7.3 ± 0.1 | 0.01 ± 0.0 |
| Coca-Cola® | Coca-Cola® (Coca-Cola Bottlers Georgia, Tbilisi, Georgia) | pH = 2.4 ± 0.1 | 2.3 ± 0.2 | |
| BF | Mousse Biorepair PERIBIOMA PRO (Coswell S.p.A., Funo di Argelato, BO, Italy) | Zinc hydroxyapatite and probiotics (Lactobacillus, bifidobacterium) | pH = 5.8 ± 0.1 | 0.03 ± 0.0 |
| BS | BUCCOTHERM (Laboratoire ODOST, Castéra-Verduzan, France) | Castéra-Verduzan Thermal Spring water | pH = 7.8 ± 0.3 | 0.01 ± 0.0 |
| WF | WATERDENT (LLC. Zelenaya dubrava, Dmitrov, Russia) | Olaflur | pH = 5.6 ± 0.2 | 0.26 ± 0.01 |
| Demographics | Values |
|---|---|
| Sex, n (%) | |
| Female | 23 (72.9) |
| Male | 9 (28.1) |
| Total | 32 (100) |
| Age | |
| Mean (sd) | 23.8 (6.1) |
| Median (Q1, Q3) | 22 (21, 23) |
| Oral Health Indicators | Values |
|---|---|
| DMFT overall, teeth | |
| Mean (sd) | 7.8 (4.8) |
| Median (Q1, Q3) | 7.5 (5.0, 10.0) |
| DT, teeth | |
| Mean (sd) | 1.3 (1.9) |
| Median (Q1, Q3) | 0 (0, 3.0) |
| MT, teeth | |
| Mean (sd) | 0.1 (0.3) |
| Median (Q1, Q3) | 0 (0, 0) |
| FT, teeth | |
| Mean (sd) | 6.2 (4.9) |
| Median (Q1, Q3) | 6.0 (2.8, 8.3) |
| OHI-S, points | |
| Mean (sd) | 1.0 (0.6) |
| Median (Q1, Q3) | 1.0 (0.5, 1.3) |
| Plaque score, points | |
| Mean (sd) | 0.9 (0.5) |
| Median (Q1, Q3) | 0.9 (0.5, 1.2) |
| Calculus score, points | |
| Mean (sd) | 0.1 (0.1) |
| Median (Q1, Q3) | 0 (0, 0.2) |
| BI, points | |
| Mean (sd) | 0.14 (0.15) |
| Median (Q1, Q3) | 0.09 (0.03, 0.17) |
| Salivary Parameters | Values |
|---|---|
| Salivation rate, mL/min | |
| Mean (sd) | 0.5 (0.2) |
| Median (Q1, Q3) | 0.4 (0.35, 0.51) |
| Salivation rate, n (%) | |
| High | 14 (43.75) |
| Medium | 14 (43.75) |
| Low | 4 (12.5) |
| Buffer capacity | |
| Mean (sd) | 6.5 (0.8) |
| Median (Q1, Q3) | 6.8 (6.0, 7.1) |
| Buffer capacity, n (%) | |
| High | 30 (93.7) |
| Medium | 2 (6.3) |
| Low | 0 (0) |
| Tested Solutions | Min pH | Clearance 1 | Area under Curve | |||
|---|---|---|---|---|---|---|
| Mean (sd) | Median (Q1; Q3) | Mean (sd) | Median (Q1; Q3) | Mean (sd) | Median (Q1; Q3) | |
| Coca-Cola® | 6.3 (0.5) a | 6.3 (5.9; 6.6) | 27.3 (15.5) A | 30 (15; 40) | 12.7 (11.0) a | 10.8 (3.5; 20.6) |
| Tap water | 6.6 (0.5) b | 6.5 (6.3; 6.8) | 17.3 (14.0) B | 15 (5; 21.3) | 7.7 (9.4) ab | 5.0 (0.6; 11.0) |
| WaterDent | 6.8 (0.6) b | 6.7 (6.4; 7.1) | 17.2 (14.8) B | 12.5 (5; 25) | 6.2 (8.3) b | 3.2 (0.4; 7.6) |
| Biorepair | 6.7 (0.6) b | 6.8 (6.4; 7.0) | 18.1 (15.0) B | 15 (5; 25) | 5.8 (9.3) b | 1.6 (0.2; 7.9) |
| Buccotherm | 6.7 (0.5) b | 6.8 (6.4; 7.1) | 15.9 (15.3) B | 10 (5; 16.3) | 7.3 (10.6) b | 2.2 (0.4; 10.7) |
| p-value 2 | 0.0000421 | 0.0225 | 0.000148 | |||
| Effect size 3 | 0.198 (small) | 0.0890 (small) | 0.177 (small) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakova, M.; Egiazaryan, A.; Doroshina, V.; Zaytsev, A.; Malashin, A.; Babina, K.; Novozhilova, N. The Effect of Oral Care Foams and a Spray on Salivary pH Changes after Exposure to Acidic Beverages in Young Adults. Dent. J. 2024, 12, 93. https://doi.org/10.3390/dj12040093
Polyakova M, Egiazaryan A, Doroshina V, Zaytsev A, Malashin A, Babina K, Novozhilova N. The Effect of Oral Care Foams and a Spray on Salivary pH Changes after Exposure to Acidic Beverages in Young Adults. Dentistry Journal. 2024; 12(4):93. https://doi.org/10.3390/dj12040093
Chicago/Turabian StylePolyakova, Maria, Anna Egiazaryan, Vladlena Doroshina, Alexandr Zaytsev, Alexey Malashin, Ksenia Babina, and Nina Novozhilova. 2024. "The Effect of Oral Care Foams and a Spray on Salivary pH Changes after Exposure to Acidic Beverages in Young Adults" Dentistry Journal 12, no. 4: 93. https://doi.org/10.3390/dj12040093