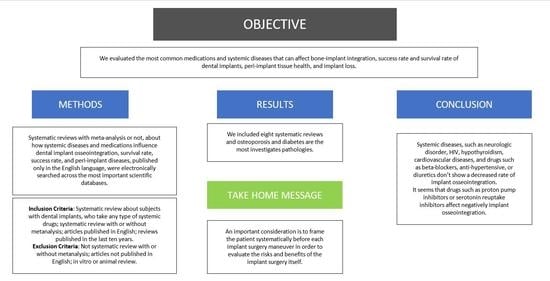
Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
- P—Population: people with dental implants taking any type of systemic drug.
- E—Exposure: effect of systemic diseases and systemic drugs.
- O—Outcomes: implant osseointegration, implant success rate, implant survival, implant loss, peri-implantitis.
2.2. Search Strategy
2.3. Study Selection and Eligibility Criteria
- -
- Systematic review about subjects with dental implants who take any type of systemic drugs.
- -
- Systematic review with or without meta-analysis.
- -
- Articles published in English.
- -
- Reviews published in the last ten years.
- -
- Not systematic review with or without meta-analysis.
- -
- Articles not published in English.
- -
- In vitro or animal review.
2.4. Data Extraction and Collection
- Author, year of publication, reference, name of the journal, and study quality;
- Number and kind of included studies;
- Characteristics of drug intake or diseases assessed;
- Main outcomes;
- Conclusions.
2.5. Data Synthesis
2.6. Assessment of Quality and Risk of Bias
3. Results
3.1. Study Selection
3.2. Studies’ Characteristics and Qualitative Synthesis
3.3. The Influence of Systemic Drug Intake or General Diseases Evaluated on the Outcomes Considered in This Umbrella Review
3.4. Quality and Risk of Bias Assessment of Included Systematic Review
4. Discussion
4.1. The Main Outcomes of This Umbrella Review (Implant Osseointegration, Implant Success Rate, Implant Survival, Peri-Implantitis, and Implant Loss)
4.1.1. Osseointegration
4.1.2. Implant Success Rate, Implant Survival Rate, Implant Loss, and Peri-implantitis
4.2. The Influence of Most Important Systemic Diseases and Drugs Evaluated on the Outcomes Considered in This Umbrella Review
4.2.1. Metabolic Bone Disease
4.2.2. Endocrine–Metabolic Disorder
4.2.3. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
4.2.4. Glucocorticoids
4.2.5. Antidepressant Medications: Selective Serotonin Reuptake Inhibitors (SSRIs)
4.2.6. Proton-Pump Inhibitors (PPIs)
4.2.7. Cardiovascular Disease
4.2.8. Neurological Disorder
4.2.9. Human Immunodeficiency Virus (HIV)
4.3. Limitations of this Study and Distorted Quality of the Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-Year Study of Osseointegrated Implants in the Treatment of the Edentulous Jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Arata, V.; Giacomello, M.; Del Fabbro, M.; Gallesio, G.; Mortellaro, C.; Bergamasco, L. Failure Risk Estimates after Dental Implants Placement Associated with Plasma Rich in Growth Factor-Endoret in Osteoporotic Women under Bisphosphonate Therapy. J. Craniofac. Surg. 2015, 26, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norcia, A.; Cicciù, M.; Matacena, G.; Bramanti, E. Dental Implant Positioning by Using the Root Way. A Predictable Technique for Postextractive Surgery. Minerva Stomatol. 2016, 65, 393–402. [Google Scholar]
- Cicciù, M.; Cervino, G.; Terranova, A.; Risitano, G.; Raffaele, M.; Cucinotta, F.; Santonocito, D.; Fiorillo, L. Prosthetic and Mechanical Parameters of the Facial Bone under the Load of Different Dental Implant Shapes: A Parametric Study. Prosthesis 2019, 1, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Sbordone, C.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Di Spirito, F. Computed Tomography-Aided Descriptive Analysis of Maxillary and Mandibular Atrophies. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 99–105. [Google Scholar] [CrossRef]
- Di Spirito, F.; Toti, P.; Brevi, B.; Martuscelli, R.; Sbordone, L.; Sbordone, C. Computed Tomography Evaluation of Jaw Atrophies before and after Surgical Bone Augmentation. Int. J. Clin. Dent. 2019, 12, 259–270. [Google Scholar]
- Jeffcoat, M. The Association between Osteoporosis and Oral Bone Loss. J. Periodontol. 2005, 76, 2125–2132. [Google Scholar] [CrossRef]
- Von Wowern, N.; Kollerup, G. Symptomatic Osteoporosis: A Risk Factor for Residual Ridge Reduction of the Jaws. J. Prosthet. Dent. 1992, 67, 656–660. [Google Scholar] [CrossRef]
- Di Spirito, F.; Lo Giudice, R.; Amato, M.; Di Palo, M.P.; D’Ambrosio, F.; Amato, A.; Martina, S. Inflammatory, Reactive, and Hypersensitivity Lesions Potentially Due to Metal Nanoparticles from Dental Implants and Supported Restorations: An Umbrella Review. Appl. Sci. 2022, 12, 11208. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. J. Dent. 2015, 43, 487–498. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Pisano, M.; Amato, A.; Iandolo, A.; Caggiano, M.; Martina, S. Periodontal and Peri-Implant Health Status in Traditional vs. Heat-Not-Burn Tobacco and Electronic Cigarettes Smokers: A Systematic Review. Dent. J. 2022, 10, 103. [Google Scholar] [CrossRef]
- Caggiano, M.; Gasparro, R.; D’Ambrosio, F.; Pisano, M.; Di Palo, M.P.; Contaldo, M. Smoking Cessation on Periodontal and Peri-Implant Health Status: A Systematic Review. Dent. J. 2022, 10, 162. [Google Scholar] [CrossRef]
- Moraschini, V.; Barboza, E. dS P. Success of Dental Implants in Smokers and Non-Smokers: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 205–215. [Google Scholar] [CrossRef]
- Ouanounou, A.; Hassanpour, S.; Glogauer, M. The Influence of Systemic Medications on Osseointegration of Dental Implants. J. Can. Dent. Assoc. 2016, 82, g7. [Google Scholar]
- Cicciù, M.; Herford, A.S.; Juodžbalys, G.; Stoffella, E. Recombinant Human Bone Morphogenetic Protein Type 2 Application for a Possible Treatment of Bisphosphonates-Related Osteonecrosis of the Jaw. J. Craniofac. Surg. 2012, 23, 784–788. [Google Scholar] [CrossRef]
- Nastro, E.; Musolino, C.; Allegra, A.; Oteri, G.; Cicciù, M.; Alonci, A.; Quartarone, E.; Alati, C.; De Ponte, F.S. Bisphosphonate-Associated Osteonecrosis of the Jaw in Patients with Multiple Myeloma and Breast Cancer. Acta Haematol. 2007, 117, 181–187. [Google Scholar] [CrossRef]
- Chadha, G.K.; Ahmadieh, A.; Kumar, S.; Sedghizadeh, P.P. Osseointegration of Dental Implants and Osteonecrosis of the Jaw in Patients Treated with Bisphosphonate Therapy: A Systematic Review. J. Oral Implantol. 2013, 39, 510–520. [Google Scholar] [CrossRef]
- Ramaglia, L.; Di Spirito, F.; Sirignano, M.; La Rocca, M.; Esposito, U.; Sbordone, L. A 5-Year Longitudinal Cohort Study on Crown to Implant Ratio Effect on Marginal Bone Level in Single Implants. Clin. Implant Dent. Relat. Res. 2019, 21, 916–922. [Google Scholar] [CrossRef]
- Di Spirito, F.; La Rocca, M.; De Bernardo, M.; Rosa, N.; Sbordone, C.; Sbordone, L. Possible Association of Periodontal Disease and Macular Degeneration: A Case-Control Study. Dent. J. 2020, 9, 1. [Google Scholar] [CrossRef]
- Di Spirito, F.; Sbordone, L.; Pilone, V.; D’Ambrosio, F. Obesity and Periodontal Disease: A Narrative Review on Current Evidence and Putative Molecular Links. Open Dent. J. 2019, 13, 526–536. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The Nuts and Bolts of PROSPERO: An International Prospective Register of Systematic Reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, L.K.; Kunz, R.; Kleijnen, J.; Antes, G. Systematic Reviews to Support Evidence-Based Medicine. How to Review and Apply Findings of Healthcare Research. K. S.; Kahn, R.; Kunz, J. Kleijnen and G. Antes. 170 × 240 mm. pp. 136. Illustrated. 2003. Royal Society of Medicine Press: London. Br. J. Surg. 2004, 91, 375. [Google Scholar]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current Methods of Preventing Aseptic Loosening and Improving Osseointegration of Titanium Implants in Cementless Total Hip Arthroplasty: A Review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef]
- De Oliveira, P.G.F.P.; Bonfante, E.A.; Bergamo, E.T.P.; de Souza, S.L.S.; Riella, L.; Torroni, A.; Benalcazar Jalkh, E.B.; Witek, L.; Lopez, C.D.; Zambuzzi, W.F.; et al. Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-Implantitis. Trends Endocrinol. Metab. 2020, 31, 596–610. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.O.; Crisan, B.; Crisan, L.; Baciut, M.; Onisor, F.; Baciut, G.; Câmpian, R.S.; Bran, S. Systemic Drugs That Influence Titanium Implant Osseointegration. Drug Metab. Rev. 2017, 49, 92–104. [Google Scholar] [CrossRef]
- Pokrowiecki, R. The Paradigm Shift for Drug Delivery Systems for Oral and Maxillofacial Implants. Drug Deliv. 2018, 25, 1504–1515. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukarasu, A.; Pinto, H.G.; Seymour, K.G. Bisphosphonate and Implant Dentistry-Is It Safe? Prim. Dent. J. 2015, 4, 30–33. [Google Scholar] [CrossRef]
- Fu, J.-H.; Bashutski, J.D.; Al-Hezaimi, K.; Wang, H.-L. Statins, Glucocorticoids, and Nonsteroidal Anti-Inflammatory Drugs: Their Influence on Implant Healing. Implant. Dent. 2012, 21, 362–367. [Google Scholar] [CrossRef]
- Basudan, A.M.; Shaheen, M.Y.; de Vries, R.B.; van den Beucken, J.J.J.P.; Jansen, J.A.; Alghamdi, H.S. Antiosteoporotic Drugs to Promote Bone Regeneration Related to Titanium Implants: A Systematic Review and Meta-Analysis. Tissue Eng. Part B Rev. 2019, 25, 89–99. [Google Scholar] [CrossRef]
- Clementini, M.; Rossetti, P.H.O.; Penarrocha, D.; Micarelli, C.; Bonachela, W.C.; Canullo, L. Systemic Risk Factors for Peri-Implant Bone Loss: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 323–334. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Subhi ALHarthi, S.; Saleh Binshabaib, M.; Javed, F. Effect of Local Zoledronate Delivery on Osseointegration: A Systematic Review of Preclinical Studies. Acta. Odontol. Scand. 2017, 75, 530–541. [Google Scholar] [CrossRef]
- Aghaloo, T.; Pi-Anfruns, J.; Moshaverinia, A.; Sim, D.; Grogan, T.; Hadaya, D. The Effects of Systemic Diseases and Medications on Implant Osseointegration: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2019, 34, s35–s49. [Google Scholar] [CrossRef]
- Chappuis, V.; Avila-Ortiz, G.; Araújo, M.G.; Monje, A. Medication-Related Dental Implant Failure: Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 55–68. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; Cicciù, M.; Tözüm, T.F.; D’Amico, C.; Oteri, G.; Cervino, G. Impact of Bisphosphonate Drugs on Dental Implant Healing and Peri-Implant Hard and Soft Tissues: A Systematic Review. BMC Oral Health 2022, 22, 291. [Google Scholar] [CrossRef]
- Luo, J.D.; Miller, C.; Jirjis, T.; Nasir, M.; Sharma, D. The Effect of Non-Steroidal Anti-Inflammatory Drugs on the Osteogenic Activity in Osseointegration: A Systematic Review. Int. J. Implant. Dent. 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between Diabetes Mellitus/Hyperglycaemia and Peri-Implant Diseases: Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Papadakis, I.; Spanou, A.; Kalyvas, D. Success Rate and Safety of Dental Implantology in Patients Treated With Antiresorptive Medication: A Systematic Review. J. Oral Implantol. 2021, 47, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Pietschmann, P.; Pandis, N.; Schiødt, M.; Klinge, B. The Effect of Antiresorptive Drugs on Implant Therapy: Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S18), 54–92. [Google Scholar] [CrossRef] [PubMed]
- Jg, W.; Sagheb, K.; Diaz, L.; Kämmerer, P.W.; Al-Nawas, B.; Schiegnitz, E. Does Vitamin D Have an Effect on Osseointegration of Dental Implants? A Systematic Review. Int. J. Implant. Dent. 2022, 8, 16. [Google Scholar] [CrossRef]
- Cheng, L.L. Systemic Intake of Proton Pump Inhibitors and Selective Serotonin Reuptake Inhibitors May Be Associated With Implant Failure. J. Evid. Based Dent. Pract. 2020, 20, 101466. [Google Scholar] [CrossRef]
- Javed, F.; Almas, K. Osseointegration of Dental Implants in Patients Undergoing Bisphosphonate Treatment: A Literature Review. J. Periodontol. 2010, 81, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Kasemo, B.; Larsson, K.; Lundström, I.; McQueen, D.H.; Skalak, R. The Interface Zone of Inorganic ImplantsIn Vivo: Titanium Implants in Bone. Ann. Biomed. Eng. 1983, 11, 1–27. [Google Scholar] [CrossRef]
- Puleo, D.A.; Nanci, A. Understanding and Controlling the Bone-Implant Interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Murai, K.; Takeshita, F.; Ayukawa, Y.; Kiyoshima, T.; Suetsugu, T.; Tanaka, T. Light and Electron Microscopic Studies of Bone-Titanium Interface in the Tibiae of Young and Mature Rats. J. Biomed. Mater. Res. 1996, 30, 523–533. [Google Scholar] [CrossRef]
- Thomsen, K.; Christensen, F.B.; Eiskjaer, S.P.; Hansen, E.S.; Fruensgaard, S.; Bünger, C.E. 1997 Volvo Award Winner in Clinical Studies. The Effect of Pedicle Screw Instrumentation on Functional Outcome and Fusion Rates in Posterolateral Lumbar Spinal Fusion: A Prospective, Randomized Clinical Study. Spine 1997, 22, 2813–2822. [Google Scholar] [CrossRef]
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant Success, Survival, and Failure: The International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Ten Bruggenkate, C.M.; van der Kwast, W.A.; Oosterbeek, H.S. Success Criteria in Oral Implantology. A Review of the Literature. Int. J. Oral Implantol. 1990, 7, 45–51. [Google Scholar]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef]
- Boccia, G.; Di Spirito, F.; D’Ambrosio, F.; Di Palo, M.P.; Giordano, F.; Amato, M. Local and Systemic Antibiotics in Peri-Implantitis Management: An Umbrella Review. Antibiotics 2023, 12, 114. [Google Scholar] [CrossRef]
- Pisano, M.; Amato, A.; Sammartino, P.; Iandolo, A.; Martina, S.; Caggiano, M. Laser Therapy in the Treatment of Peri-Implantitis: State-of-the-Art, Literature Review and Meta-Analysis. Appl. Sci. 2021, 11, 5290. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; De Caro, F.; Lanza, A.; Passarella, D.; Sbordone, L. Adherence to Antibiotic Prescription of Dental Patients: The Other Side of the Antimicrobial Resistance. Healthcare 2022, 10, 1636. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Di Spirito, F.; Amato, A.; Caggiano, M.; Lo Giudice, R.; Martina, S. Attitudes towards Antibiotic Prescription and Antimicrobial Resistance Awareness among Italian Dentists: What Are the Milestones? Healthcare 2022, 10, 1585. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; Jansen, J.A. Bone Regeneration Associated with Nontherapeutic and Therapeutic Surface Coatings for Dental Implants in Osteoporosis. Tissue Eng. Part B Rev. 2013, 19, 233–253. [Google Scholar] [CrossRef]
- Radi, I.A.-W.; Ibrahim, W.; Iskandar, S.M.S.; AbdelNabi, N. Prognosis of Dental Implants in Patients with Low Bone Density: A Systematic Review and Meta-Analysis. J. Prosthet Dent. 2018, 120, 668–677. [Google Scholar] [CrossRef]
- Lazarovici, T.S.; Yahalom, R.; Taicher, S.; Schwartz-Arad, D.; Peleg, O.; Yarom, N. Bisphosphonate-Related Osteonecrosis of the Jaw Associated with Dental Implants. J. Oral Maxillofac. Surg. 2010, 68, 790–796. [Google Scholar] [CrossRef]
- Ferreira Jr, L.H.; Mendonça Jr, K.D.; Chaves de Souza, J.; Soares Dos Reis, D.C.; do Carmo Faleiros Veloso Guedes, C.; de Souza Castro Filice, L.; Bruzadelli Macedo, S.; Soares Rocha, F. Bisphosphonate-Associated Osteonecrosis of the Jaw. Minerva. Dent. Oral Sci. 2021, 70, 49–57. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- DeBaz, C.; Hahn, J.; Lang, L.; Palomo, L. Dental Implant Supported Restorations Improve Quality of Life in Osteoporotic Women. Int. J. Dent. 2015, 2015, 451923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, M.A.; Rashid, F.; Feine, J.S. Overdenture Effectiveness Study Team Consortium The Effect of Mandibular 2-Implant Overdentures on Oral Health-Related Quality of Life: An International Multicentre Study. Clin. Oral Implant. Res. 2014, 25, 46–51. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Hasan, S.M.; Khan, R.S. Bisphosphonate Releasing Dental Implant Surface Coatings and Osseointegration: A Systematic Review. J. Taibah. Univ. Med. Sci. 2017, 12, 369–375. [Google Scholar] [CrossRef]
- De Medeiros, F.C.F.L.; Kudo, G.a.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental Implants in Patients with Osteoporosis: A Systematic Review with Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Zahid, T.M.; Wang, B.-Y.; Cohen, R.E. Influence of Bisphosphonates on Alveolar Bone Loss around Osseointegrated Implants. J. Oral Implantol. 2011, 37, 335–346. [Google Scholar] [CrossRef]
- Kasai, T.; Pogrel, M.A.; Hossaini, M. The Prognosis for Dental Implants Placed in Patients Taking Oral Bisphosphonates. J. Calif. Dent. Assoc. 2009, 37, 39–42. [Google Scholar] [CrossRef]
- Caggiano, M.; D’Ambrosio, F.; Giordano, F.; Acerra, A.; Sammartino, P.; Iandolo, A. The “Sling” Technique for Horizontal Guided Bone Regeneration: A Retrospective Case Series. Appl. Sci. 2022, 12, 5889. [Google Scholar] [CrossRef]
- Yip, J.K.; Borrell, L.N.; Cho, S.-C.; Francisco, H.; Tarnow, D.P. Association between Oral Bisphosphonate Use and Dental Implant Failure among Middle-Aged Women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Robinson, F.G.; Romanos, G.; Thomas, M.V. Osteoporosis and Bisphosphonate-Related Osteonecrosis in a Dental School Implant Patient Population. Implant. Dent. 2015, 24, 328–332. [Google Scholar] [CrossRef]
- Di Spirito, F.; Argentino, S.; Martuscelli, R.; Sbordone, L. Mronj Incidence After Multiple Teeth Extractions in Patients Taking Oral Bis-Phosphonates Without “Drug Holiday”: A Retrospective Chart Review. Oral Implantol. 2019, 12, 105–110. [Google Scholar]
- Lau, H.K.; Mounsey, A.; Mackler, L. Human Parathyroid Hormone for Treating Osteoporosis. Am. Fam. Physicians 2012, 85. [Google Scholar]
- Tao, Z.-S.; Zhou, W.-S.; Qiang, Z.; Tu, K.; Huang, Z.-L.; Xu, H.-M.; Sun, T.; Lv, Y.-X.; Cui, W.; Yang, L. Intermittent Administration of Human Parathyroid Hormone (1–34) Increases Fixation of Strontium-Doped Hydroxyapatite Coating Titanium Implants via Electrochemical Deposition in Ovariectomized Rat Femur. J. Biomater. Appl. 2016, 30, 952–960. [Google Scholar] [CrossRef]
- Di Spirito, F.; Schiavo, L.; Pilone, V.; Lanza, A.; Sbordone, L.; D’Ambrosio, F. Periodontal and Peri-Implant Diseases and Systemically Administered Statins: A Systematic Review. Dent. J. 2021, 9, 100. [Google Scholar] [CrossRef]
- Javed, F.; Al Amri, M.D.; Kellesarian, S.V.; Al-Kheraif, A.A.; Vohra, F.; Calvo-Guirado, J.L.; Malmstrom, H.; Romanos, G.E. Efficacy of Parathyroid Hormone Supplementation on the Osseointegration of Implants: A Systematic Review. Clin. Oral Investig. 2016, 20, 649–658. [Google Scholar] [CrossRef]
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jané-Salas, E.; Viñas, M.; López-López, J. Oral Manifestations of Diabetes Mellitus. A Systematic Review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e586–e594. [Google Scholar] [CrossRef]
- Amato, M.; Zingone, F.; Caggiano, M.; Iovino, P.; Bucci, C.; Ciacci, C. Tooth Wear Is Frequent in Adult Patients with Celiac Disease. Nutrients 2017, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental Implants and Diabetes Mellitus-a Systematic Review. Int. J. Implant. Dent. 2016, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-Implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S246–S266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.D.; Silva, G.L.M.; Cortelli, J.R.; Costa, J.E.; Costa, F.O. Prevalence and Risk Variables for Peri-Implant Disease in Brazilian Subjects. J. Clin. Periodontol. 2006, 33, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Tawil, G.; Younan, R.; Azar, P.; Sleilati, G. Conventional and Advanced Implant Treatment in the Type II Diabetic Patient: Surgical Protocol and Long-Term Clinical Results. Int. J. Oral Maxillofac. Implant. 2008, 23, 744–752. [Google Scholar]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.M.; Ferreira, S.D.; Silva, G.L.M.; Costa, J.E. Peri-Implant Disease in Subjects with and without Preventive Maintenance: A 5-Year Follow-Up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.-M.; Renvert, H.; Lindahl, C.; Renvert, S. Nine- to Fourteen-Year Follow-up of Implant Treatment. Part III: Factors Associated with Peri-Implant Lesions. J. Clin. Periodontol. 2006, 33, 296–301. [Google Scholar] [CrossRef]
- Máximo, M.B.; de Mendonça, A.C.; Alves, J.F.; Cortelli, S.C.; Peruzzo, D.C.; Duarte, P.M. Peri-Implant Diseases May Be Associated with Increased Time Loading and Generalized Periodontal Bone Loss: Preliminary Results. J. Oral Implantol. 2008, 34, 268–273. [Google Scholar] [CrossRef]
- Renvert, S.; Aghazadeh, A.; Hallström, H.; Persson, G.R. Factors Related to Peri-Implantitis–A Retrospective Study. Clin. Oral Implant. Res. 2014, 25, 522–529. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of Diabetes Mellitus and Glycemic Control on the Osseointegration of Dental Implants: A Systematic Literature Review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.R.; Bassett, J.H.D. Thyroid Diseases and Bone Health. J. Endocrinol. Investig. 2018, 41, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Schmid, C.; Schläpfer, I.; Futo, E.; Waldvogel, M.; Schwander, J.; Zapf, J.; Froesch, E.R. Triiodothyronine (T3) Stimulates Insulin-like Growth Factor (IGF)-1 and IGF Binding Protein (IGFBP)-2 Production by Rat Osteoblasts in Vitro. Acta Endocrinol. 1992, 126, 467–473. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical Pharmacology of Non-Steroidal Anti-Inflammatory Drugs: A Review. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Chikazu, D.; Tomizuka, K.; Ogasawara, T.; Saijo, H.; Koizumi, T.; Mori, Y.; Yonehara, Y.; Susami, T.; Takato, T. Cyclooxygenase-2 Activity Is Essential for the Osseointegration of Dental Implants. Int. J. Oral Maxillofac. Surg. 2007, 36, 441–446. [Google Scholar] [CrossRef]
- Alissa, R.; Sakka, S.; Oliver, R.; Horner, K.; Esposito, M.; Worthington, H.V.; Coulthard, P. Influence of Ibuprofen on Bone Healing around Dental Implants: A Randomised Double-Blind Placebo-Controlled Clinical Study. Eur. J. Oral Implantol. 2009, 2, 185–199. [Google Scholar]
- Winnett, B.; Tenenbaum, H.C.; Ganss, B.; Jokstad, A. Perioperative Use of Non-Steroidal Anti-Inflammatory Drugs Might Impair Dental Implant Osseointegration. Clin. Oral Implant. Res. 2016, 27, e1–e7. [Google Scholar] [CrossRef]
- Smith, R.A.; Berger, R.; Dodson, T.B. Risk Factors Associated with Dental Implants in Healthy and Medically Compromised Patients. Int. J. Oral Maxillofac. Implant. 1992, 7, 367–372. [Google Scholar]
- Cranin, A.N. Endosteal Implants in a Patient with Corticosteroid Dependence. J. Oral Implantol. 1991, 17, 414–417. [Google Scholar]
- Beumer, J.; Landesman, H.; Terry, B.C.; Davis, W.H.; Davis, C.L. Prosthodontic and Surgical Aspects of Treatment Planning for Reconstructive Surgery. In Reconstructive Preprosthetic Oral and Maxillofacial Surgery, 2nd ed.; Fonseca, R.J., Davis, W.H., Eds.; Saunders: Philadelphia, PA, USA, 1986; pp. 41–60. [Google Scholar]
- Fujimoto, T.; Niimi, A.; Sawai, T.; Ueda, M. Effects of Steroid-Induced Osteoporosis on Osseointegration of Titanium Implants. Int. J. Oral Maxillofac. Implants 1998, 13, 183–189. [Google Scholar]
- D’Ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic Stress and Depression in Periodontitis and Peri-Implantitis: A Narrative Review on Neurobiological, Neurobehavioral and Immune-Microbiome Interplays and Clinical Management Implications. Dent. J. 2022, 10, 49. [Google Scholar] [CrossRef]
- Scandurra, C.; Gasparro, R.; Dolce, P.; Bochicchio, V.; Muzii, B.; Sammartino, G.; Marenzi, G.; Maldonato, N.M. The Role of Cognitive and Non-Cognitive Factors in Dental Anxiety: A Mediation Model. Eur. J. Oral Sci. 2021, 129, e12793. [Google Scholar] [CrossRef]
- Diem, S.J.; Blackwell, T.L.; Stone, K.L.; Yaffe, K.; Haney, E.M.; Bliziotes, M.M.; Ensrud, K.E. Use of Antidepressants and Rates of Hip Bone Loss in Older Women: The Study of Osteoporotic Fractures. Arch. Intern. Med. 2007, 167, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- Haney, E.M.; Chan, B.K.S.; Diem, S.J.; Ensrud, K.E.; Cauley, J.A.; Barrett-Connor, E.; Orwoll, E.; Bliziotes, M.M. Osteoporotic Fractures in Men Study Group Association of Low Bone Mineral Density with Selective Serotonin Reuptake Inhibitor Use by Older Men. Arch. Intern. Med. 2007, 167, 1246–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Bencaz, A.F.; Hentz, J.G.; Crowell, M.D. Selective Serotonin Reuptake Inhibitor Treatment and Risk of Fractures: A Meta-Analysis of Cohort and Case-Control Studies. Osteoporos. Int. 2012, 23, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, E.M.; Gamie, Z.; Tran, G.T.; Adshead, S.; Lampard, A.; Mantalaris, A.; Tsiridis, E. The Adverse Skeletal Effects of Selective Serotonin Reuptake Inhibitors. Eur. Psychiatry 2012, 27, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Al-Abedalla, K.; Rastikerdar, E.; Abi Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Selective Serotonin Reuptake Inhibitors and the Risk of Osseointegrated Implant Failure: A Cohort Study. J. Dent. Res. 2014, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, B.K.; Cohen, R.E.; Stellrecht, E.M.; Yerke, L.M. The Influence of Proton Pump Inhibitors on Tissue Attachment around Teeth and Dental Implants: A Scoping Review. Clin. Exp. Dent. Res. 2022, 8, 1045–1058. [Google Scholar] [CrossRef]
- Vinnakota, D.N.; Kamatham, R. Effect of Proton Pump Inhibitors on Dental Implants: A Systematic Review and Meta-Analysis. J. Indian Prosthodont. Soc. 2020, 20, 228–236. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Eimar, H.; Arekunnath Madathil, S.; Abi-Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Antihypertensive Medications and the Survival Rate of Osseointegrated Dental Implants: A Cohort Study. Clin. Implant. Dent. Relat. Res. 2016, 18, 1171–1182. [Google Scholar] [CrossRef]
- Borderi, M.; Gibellini, D.; Vescini, F.; De Crignis, E.; Cimatti, L.; Biagetti, C.; Tampellini, L.; Re, M.C. Metabolic Bone Disease in HIV Infection. AIDS 2009, 23, 1297–1310. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Gallottini, M.; Pallos, D.; Maluf, P.S.Z.; Jablonka, F.; Ortega, K.L. The Success of Endosseous Implants in Human Immunodeficiency Virus-Positive Patients Receiving Antiretroviral Therapy: A Pilot Study. J. Am. Dent. Assoc. 2011, 142, 1010–1016. [Google Scholar] [CrossRef]
- Ata-Ali, J.; Ata-Ali, F.; Di-Benedetto, N.; Bagán, L.; Bagán, J.-V. Does HIV Infection Have an Impact upon Dental Implant Osseointegration? A Systematic Review. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e347–e356. [Google Scholar] [CrossRef]
- Amato, M.; Di Spirito, F.; D’Ambrosio, F.; Boccia, G.; Moccia, G.; De Caro, F. Probiotics in Periodontal and Peri-Implant Health Management: Biofilm Control, Dysbiosis Reversal, and Host Modulation. Microorganisms 2022, 10, 2289. [Google Scholar] [CrossRef]



| Authors, Year | Motivation |
|---|---|
| Apostu, 2018 [27] | No systematic review |
| De Oliveira, 2020 [28] | No systematic review |
| Apostu, 2017 [29] | No systematic review |
| Pokrowiecki, 2018 [30] | Not relevant |
| Thirunavukarasu, 2015 [31] | Not relevant |
| Ouanounou, 2016 [15] | Did not meet the inclusion criteria |
| Fu, 2012 [32] | No systematic review |
| Basudan, 2018 [33] | No human studies |
| Clementini, 2013 [34] | Not relevant |
| Kellesarian, 2017 [35] | Did not meet the inclusion criteria |
| Author, Year Reference Journal Meta-Analysis | Number and Design of Included Studies | Type of Drug Intake or Diseases Evaluated | Outcomes | Conclusions |
|---|---|---|---|---|
| Aghaloo, 2019 [36]; Int J Oral Maxillofacial Implants; systematic review | Osteoporosis, not taking BPs: 2 PSs, 2 CCSs Osteoporosis, taking BPs: 10 RSs, 3 CCSs, 4 PSs Diabetes: 5 RSs, 5 CCSs, 10 PSs | Osteoporosis, diabetes, cardiovascular disease or hypertension, Parkinson’s disease or neurocognitive disease, rheumatoid arthritis, hypothyroidism, HIV, depression, anti-hypertensives or diuretics, PPIs, and SSRIs | Implant osseointegration | It seems that patients with osteoporosis, diabetes, hypothyroidism, HIV, neurocognitive disease, rheumatoid arthritis, and cardiovascular disease do not have a decreasing rate of implant osseointegration. Instead, SSRIs and PPIs show a negative effect on osseointegration. |
| Chappuis, 2018 [37]; Clinical Oral Implants Research; systematic review and meta-analysis | NSAIDs: 5 RCTs, 2 RSs SSRI: 2 RSs PPI: 2 RSs BP: 5 RSs, 1 CCS, 1 PS; AHTN: 1 RS | BP, NSAIDs, SSRIs, PPIs, AHTNs | Implant failure, MIBL | The authors show that PPIs and SSRIs negatively influence implant success and oral BPs did not yield significance upon implant failure. |
| Fiorillo, 2022 [38]; BMC Oral Health; systematic review | 5 RCTs, 3 MSs, 1 CT | BPs | BoP, PPD, MIBL, mobility of dental implant, ISQ, BMD, implant survival, TE, soft tissue condition | The authors underline that there are no statistically significant differences in MIBL, despite the fact that implants associated with BPs show better results. The authors underline pharmacological prophylaxis before implant insertion. |
| Luo, 2018 [39]; International Journal of Implant Dentistry; systematic review | 2 in vitro studies, 3 CTs, 8 animal studies | NSAIDs | Implant osseointegration | NSAIDs do not negatively influence osseointegration in human studies, although these results contrast with in vitro and in vivo animal studies. |
| Monje, 2017 [40]; Journal of Clinical Periodontology; systematic review and meta-analysis | 5 PSs, 1 RSs, 6 CSSs | Hyperglycemia | Peri-implant mucositis, peri-implantitis | The risk of peri-implantitis is higher in hyperglycemic subjects than normoglycemic subjects but with not statistically significance differences. |
| Papadakis, 2023 [41]; J Oral Implantology; systematic review | 4 PSs, 7 CCSs 21 RSs | ARDs | Success rate, survival rates | This review describes that ARDs do not influence the success and survival rates of dental implants. |
| Stavropoulos, 2018 [42]; Clinical Oral Implants Research; systematic review and meta-analysis | BP intake: 8 CSs, 10 cohort studies, 6 CCSs HRT intake: 5 CSs, 2 CCSs MRONJ associated with implants: 7 CSs | ARDs, BPs, HRT | Implant loss, failure of grafting procedure, MIBL, MRONJ, peri-implantitis | This review showed that low-dose oral BPs do not compromise implants and do not show complications/ failures as compared to patients with no BP intake. High-dose BPs or other ARDs show a high risk for MRONJ, but few studies are about implant therapy. |
| Werny, 2022 [43] International Journal of Implant Dentistry; systematic review | 13 animal studies, 3 CTs, 2 human RSs | Vitamin D | Implant osseointegration | Vitamin D deficiency negatively influences osseointegration and its supplementation improves osseointegration in animals. Limited information is available for human implant osseointegration. |
| Outcomes | Author, Year | Drug Intake and General Diseases | Main Result |
|---|---|---|---|
| Osseointegration | Aghaloo (2019) [36] | ARDs | No differences in patients with or without osteoporosis with antiresorptive therapy. |
| Diabetes | No differences were seen among diabetic and healthy patients. | ||
| Luo et al. (2018) [39] | NSAIDs | Could not be adequately estimated. | |
| Werny et al. (2022) [43] | Vitamin D | There was slight evidence that vitamin D supplementation improves implant osseointegration in humans. | |
| Implant survival rate | Aghaloo et al. (2019) [36] | Osteoporosis | The pooled estimated was 98% (with a confidence interval of 95%). |
| Neurologic disorder | The implant survival rate was 86%. | ||
| Papadakis et al. (2023) [41] | ARDs | Antiresorptive medication did not reduce the success rate of dental implants or implant survival rates. | |
| Chappuis et al. (2018) [37] | Anti-hypertensive, diuretics, or beta blockers | The analysis could not be performed. | |
| Implant loss rate | Stavropoulos et al. (2018) [42] | ARDs | No differences in patients with or without osteoporosis with antiresorptive therapy. |
| HRT | HRT intake studies reported a higher implant loss rate (9.1–27.3%) compared to controls (7.4–16.1%). | ||
| Implant success rate | Papadakis et al. (2023) [41] | ARDs | ARDs did not reduce the success rate of dental implants or implant survival rates. |
| Implant failure rate | Chappuis et al. (2018) [37] | ARDs | No differences in patients with or without osteoporosis with antiresorptive therapy. |
| Anti-hypertensive, diuretics, or beta blockers | The analysis could not be performed. | ||
| SSRIs | It seems that the test (SSRI intake) group had a significantly higher risk than the control group. | ||
| PPIs | Implant failure rates were higher in the test group compared to the control group (p < 0.01). | ||
| Aghaloo et al. (2019) [36] | HIV | A 0.8% failure rate compared to 100% failure in non-HIV patients. | |
| Cardiovascular disease | No statistically significant differences were found among test and control groups. | ||
| Hypothyroidism | No differences were found among healthy and diseased patients. | ||
| Anti-hypertensive, diuretics, or beta blockers | In patients who took medication, implant survival rate was 99.4% versus 95.9% in patients not taking these medications. | ||
| SSRIs | A lower implant survival rate of 89.4% to 94.4% vs. 95.4% to 98.15% in patients not taking these drugs was shown. | ||
| PPIs | PPI users had an increased implant failure rate of 12% to 6.8% vs. 4.5% to 3.2% in PPI non-users. | ||
| Bone marginal loss | Fiorillo et al. (2022) [38] | ARDs | No differences in patients with or without osteoporosis with antiresorptive therapy. |
| Peri-implant mucositis or peri-implantitis | Monje et al. (2017) [40] | Diabetes | The risk of peri-implantitis in hyperglycemic subjects was statistically significantly higher than normoglycemic subjects. |
| Level | Description | Aghaloo, 2019 [36] | Chappuis, 2018 [37] | Fiorillo, 2022 [38] | Luo, 2018 [39] | Monje, 2017 [40] | Papadakis, 2023 [41] | Stavropoulos, 2018 [42] | Werny, 2022 [43] |
|---|---|---|---|---|---|---|---|---|---|
| High | No or one non-critical weakness: the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest. | ||||||||
| Moderate | More than one non-critical weakness: The systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review. | ✓ | ✓ | ||||||
| Low | One critical flaw with or without non-critical weaknesses: the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest. | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Critically low | More than one critical flaw with or without non-critical weaknesses: the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies. | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dent. J. 2023, 11, 146. https://doi.org/10.3390/dj11060146
D’Ambrosio F, Amato A, Chiacchio A, Sisalli L, Giordano F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dentistry Journal. 2023; 11(6):146. https://doi.org/10.3390/dj11060146
Chicago/Turabian StyleD’Ambrosio, Francesco, Alessandra Amato, Andrea Chiacchio, Laura Sisalli, and Francesco Giordano. 2023. "Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review" Dentistry Journal 11, no. 6: 146. https://doi.org/10.3390/dj11060146